- Home
- »
- Medical Devices
- »
-
Insulin Delivery Devices Market Size And Share Report, 2030GVR Report cover
![Insulin Delivery Devices Market Size, Share & Trends Report]()
Insulin Delivery Devices Market (2024 - 2030) Size, Share & Trends Analysis Report By Product (Reusable Insulin Pens, Disposable Insulin Pens), By End Use (Hospitals & Clinics, Homecare), By Region, And Segment Forecasts
- Report ID: GVR-1-68038-991-3
- Number of Report Pages: 150
- Format: PDF
- Historical Range: 2018 - 2022
- Forecast Period: 2024 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Interactive Charts
- Methodology
- Download FREE Sample
-
Download Sample Report
Insulin Delivery Devices Market Summary
The global insulin delivery devices market size was estimated at USD 16.42 billion in 2023 and is projected to reach USD 28.06 billion by 2030, growing at a CAGR of 7.89% from 2024 to 2030. The surge in market growth is largely attributed to the rising global prevalence of diabetes, which has heightened the demand for advanced and user-friendly solutions for diabetes management.
Key Market Trends & Insights
- The North America insulin delivery devices market held the largest share of 40.73% in 2023.
- The insulin delivery devices market in the U.S. held the largest market share in 2023 in the North America region.
- By product, insulin pens have captured the largest market share of 43.68% in 2023.
- By end use, the hospitals and clinics segment is expected to witness the fastest CAGR of 8.42% during the forecast period.
Market Size & Forecast
- 2023 Market Size: USD 16.42 Billion
- 2030 Projected Market Size: USD 28.06 Billion
- CAGR (2024-2030): 7.89%
- North America: Largest market in 2023
Technological advancements play a crucial role in the market growth, with the development of devices that are more effective, less invasive, and tailored to meet patients' individual needs.
Furthermore, integrating digital technology with insulin delivery devices is expected to boost market growth. This includes the rise of Continuous Glucose Monitoring (CGM) systems that work in tandem with insulin delivery devices to provide real-time glucose monitoring and automated insulin adjustments.
Technological advancements in diabetes management have significantly transformed insulin delivery methods, evolving from traditional injections to more advanced, minimally invasive techniques. Initially, insulin administration faced challenges in maintaining consistent blood glucose levels. However, the advent of insulin pens facilitated easier self-administration, reducing discomfort. The development of insulin pumps allowed for continuous insulin infusion, enhancing glucose control. A pivotal advancement was the introduction of Continuous Glucose Monitoring (CGM) devices, providing real-time glucose data, essential for informed decision-making. This set the stage for Artificial Pancreas Systems (APS), which integrate CGM data with insulin pumps for automated delivery, closely mimicking natural pancreatic function. Bionic technology, blending biological and artificial processes, has been instrumental in advancing insulin delivery. It underpins devices such as APS, utilizing algorithms, sensors, and automated pumps for precise blood sugar regulation, minimizing manual intervention. These innovations offer tailored, user-friendly solutions, notably improving patient outcomes. Modern insulin pumps, equipped with CGM integration, predictive algorithms, and smartphone connectivity, exemplify significant progress in device functionality, offering accurate and adaptable insulin management. These features not only mitigate risks associated with blood glucose extremes but also empower users with finer control over their insulin dosing, responding dynamically to glucose level changes.
The evolution towards bionic insulin delivery systems underscores a shift towards automation, precision, and enhanced safety in diabetes management. By significantly reducing the incidence of hypoglycemia and enabling stable glucose control, these devices contribute to improved health outcomes and quality of life for individuals with diabetes. The continuous innovation in bionic technology and its integration into diabetes care is poised to catalyze growth in the insulin delivery devices industry. The emphasis on accuracy, adaptability, and user-friendliness in device design, coupled with the potential for automated, personalized diabetes management, signals a promising future for market expansion.
Furthermore, the insulin delivery devices industry is witnessing a shift towards wearable devices, such as insulin pumps and insulin patch pumps, which offer continuous insulin infusion in a convenient and discreet manner. For instance, In January 2024, PharmaSens, a Switzerland-based company specializing in medical devices, has recently applied for FDA approval in the United States for its 'niia essential' insulin patch pump system. This application represents a pivotal step for PharmaSens in the evolution of insulin pump technologies. Furthermore, the company achieved ISO 13485 certification in November 2023, encompassing the design, development, manufacturing, and distribution of insulin infusion pumps and their accessories. PharmaSens has positioned its product as focused on patient needs and ease of use. Furthermore, In January 2024, Embecta Corp. declared that it had submitted a 510(k) premarket application to the U.S. Food and Drug Administration (FDA) for its unique disposable insulin delivery system.
"This patch pump is intended for people who require insulin to manage diabetes and is informed by the unique needs of people with type 2 diabetes and their healthcare providers, We worked with them to address the needs of those who may require more daily insulin and are looking for a simplified and convenient option for automated insulin delivery that offers the ease of use and discretion of a patch pump, along with a larger 300U insulin reservoir."
-said Colleen Riley, Chief Technology Officer, embecta.
The development of smart insulin pens, which can track and share dosage data, represents another significant trend, as does the progression towards bionic and artificial pancreas systems that more closely mimic the natural insulin delivery of a healthy pancreas. For instance, in March 2022, Novo Nordisk introduced two smart connected insulin pens, the NovoPen 6 and NovoPen Echo Plus, which are accessible via prescription for individuals with diabetes in the UK, treated with Novo Nordisk's insulin. These advanced insulin pens have the capability to log details of insulin doses, such as the timing and quantity of insulin administered. The collected data can then be synchronized with a compatible application, allowing both individuals with diabetes and their healthcare providers to review the information.
The rising geriatric population is fueling the insulin delivery devices industry growth. These devices, such as insulin pens, pumps, and smart insulin devices, cater specifically to the needs of older adults by simplifying the insulin administration process, which can be particularly challenging due to age-related factors such as reduced vision, skill, and cognitive function. The ease of use, dosing accuracy, and the ability to integrate with digital health platforms for monitoring and reminders ensure better adherence to treatment regimens, thereby improving glycemic control and reducing the risk of complications. Given the increasing prevalence of diabetes among older adults globally, the growth potential within this demographic is substantial. Manufacturers continuously innovate to make devices more geriatric-friendly by incorporating features such as larger, easy-to-read displays, simple interfaces, and minimal manual dexterity requirements. These characteristics significantly contribute to the larger adoption of insulin delivery devices by the aging population, making them a crucial aspect of diabetes care in old age and highlighting their expanding role in the management of diabetes among the elderly.
Market Concentration & Characteristics
The market is characterized by a moderate to high level of industry concentration, with a few major global players dominating the scene. These include companies such as Novo Nordisk, Sanofi, Eli Lilly, Medtronic, and Insulet Corporation. These companies have established strong brand recognition, extensive product lines, and significant global footprints. The market is driven by the rising prevalence of diabetes worldwide, increasing awareness about diabetes management, and the continuous need for more effective and convenient insulin delivery methods.
Innovation is a cornerstone of the insulin delivery devices industry. Companies are continually researching and developing new technologies to make insulin delivery more efficient, less painful, and more user-friendly. Recent innovations include smart insulin pens that can track dosage and timing, patch pumps that adhere to the skin without the need for tubing, and closed-loop systems that adjust insulin delivery in real-time based on continuous glucose monitoring data. In January 2024, Medtronic plc received the CE Mark approval for its MiniMed 780G system, which includes the Simplera Sync, a new, single-use, integrated continuous glucose monitor (CGM) that eliminates the need for fingersticks or additional adhesive. The Simplera Sync offers an enhanced user experience with an easy two-step setup and is significantly smaller than Medtronic's previous sensor models. The MiniMed 780G system, equipped with the Simplera Sync sensor, is set to be introduced in Europe through a select release in the spring of 2024. Following this, Medtronic plans to start a gradual rollout across Europe in the summer of 2024. Currently, the MiniMed 780G system is compatible with the Guardian 4 sensor.
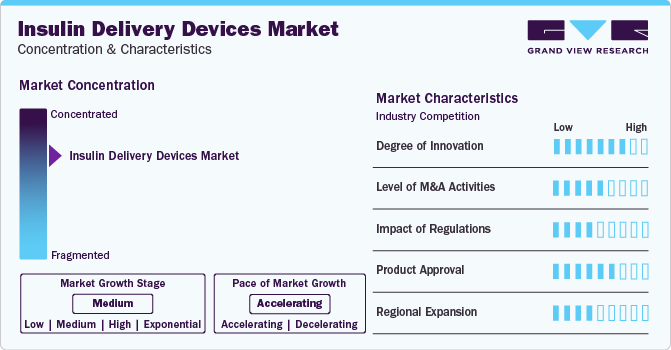
The market has witnessed a steady level of mergers and acquisitions (M&A) activities as companies strive to enhance their product portfolios, expand their market reach, and leverage new technologies. M&A activities enable larger companies to quickly adopt innovative technologies developed by smaller firms and startups, thereby accelerating the pace of innovation within the industry. In February 2024, Novo Nordisk entered into an agreement to purchase three fill-finish facilities from Novo Holdings A/S (Novo Holdings), as part of a deal that also sees Novo Holdings acquiring Catalent, Inc. (Catalent), a leading global contract development and manufacturing organization based in Somerset, New Jersey (US). This move further strengthens the long-established partnership between Novo Nordisk and Catalent. By acquiring these fill-finish sites, Novo Nordisk aims to extend its reach to more individuals suffering from diabetes and obesity with its existing and future therapies. This strategic acquisition will enhance the company's manufacturing capabilities, allowing for rapid and extensive scale-up, while also providing additional flexibility and options within Novo Nordisk’s current supply chain. The impact of this acquisition will start to significantly boost Novo Nordisk's fill-finish capacity beginning in 2026.
Regulatory approvals are crucial in the insulin delivery devices industry. The process can be lengthy and requires substantial evidence of safety and efficacy. Regulations can significantly impact the time-to-market of new devices. However, regulatory bodies like the FDA in the United States and the EMA in Europe have been working towards streamlining the approval process for diabetes management devices, recognizing their critical role in patient care. In February 2024, Insulet Corporation announced the attainment of the CE mark approval in accordance with the European Medical Device Regulation. This approval is for the enhanced compatibility with the Abbott FreeStyle Libre 2 Plus sensor of Insulet’s Omnipod 5 Automated Insulin Delivery System, designed for use by individuals as young as two years old who are living with type 1 diabetes. The Omnipod 5 stands out as the inaugural tubeless hybrid closed-loop system, also known as an automated insulin delivery system, that has earned CE mark approval. Uniquely, it integrates with two CGM sensor brands: Abbott FreeStyle Libre and Dexcom. The system comprises a tubeless Pod, which is optimized with SmartAdjust Technology, and the Omnipod 5 Controller, featuring an integrated Smartbolus Calculator.
Product approvals are a key driver for growth and competition in the market. Companies are in a constant race to get their new and improved devices approved by regulatory authorities. These approvals not only validate the safety and efficacy of the devices but also often bring about significant improvements in diabetes management for patients. For instance, Sanofi (India) has obtained marketing approval from the CDSCO for its diabetes medication, Soliqua, available in pre-filled pens in March 2023. Designed for adults with type 2 diabetes and obesity who have not witnessed sufficient control with other therapies, Soliqua enhances blood sugar levels alongside diet and exercise. It is administered once daily in a set combination (10-40 and 30-60) of insulin glargine and lixisenatide.
The global nature of diabetes as a health issue means that there is a demand for insulin delivery devices across the world. However, market penetration varies by region, influenced by factors such as healthcare infrastructure, regulatory environment, and economic conditions. Companies are continuously exploring opportunities to expand into emerging markets, where the prevalence of diabetes is growing rapidly, alongside efforts to consolidate their presence in established markets. For instance, starting in 2023, Novo Nordisk has disclosed an investment exceeding 16 billion Danish kroner (approximately 2.1 billion euros) aimed at enlarging its existing production facility in Chartres, France, in November 2023. This expansion is intended to cater to both present and future product lines targeting severe chronic conditions. With this investment, the production site's capabilities will be substantially enhanced by the incorporation of aseptic production, the completion of production processes, and the expansion of the existing Quality Control Laboratory. Including provisions for GLP-1 product manufacturing, this financial commitment is set to bolster Novo Nordisk's capacity to supply innovative treatments in response to evolving healthcare needs.
Product Insights
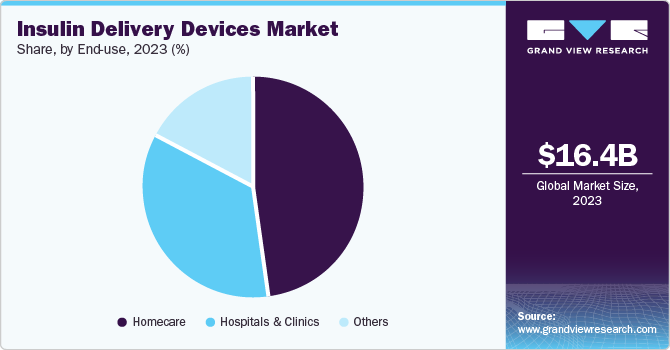
Insulin pens have captured the largest market share of 43.68% in 2023. These pen-like devices are designed to deliver insulin in a convenient, accurate, and less invasive manner. They are particularly beneficial for individuals with diabetes who require regular insulin injections. For instance, as per data released by MSF-SA Association in May 2024, a survey conducted by MSF and T1International involving 38 countries and over 400 individuals using insulin revealed that 82% of respondents who had experience with both insulin pens and traditional syringes expressed a preference for insulin pens. The preference was attributed to several factors, including the ease and safety of pen usage, accurate dosing, reduced pain during administration, and decreased social stigma when using pens in public settings. These advantages contribute to enhanced quality of life and greater adherence to insulin treatment protocols. Reusable insulin pens have captured the largest market share among the insulin pens segment. Some of the commercially available reusable insulin pens include AllStar, ClikSTAR, JuniorSTAR, and TouStar by Sanofi.
The insulin pumps segment is expected to grow at the fastest CAGR during the forecast period. Insulin pumps provide a consistent and accurate way to deliver insulin to people with diabetes. These compact, wearable gadgets deliver insulin through a tiny tube placed beneath the skin, replicating the natural function of a healthy pancreas. They offer a steady supply of basal insulin throughout the day and additional doses, known as boluses, to match mealtime requirements. A study published by the American Diabetes Association in October 2023 revealed a growing trend in insulin pump usage over time, with variations noted based on gender, insurance coverage, and racial/ethnic backgrounds. Those using insulin pumps showed improved A1C levels, increased adoption of Continuous Glucose Monitoring (CGM), and reduced occurrences of diabetic ketoacidosis and severe hypoglycemia. Individuals utilizing insulin pumps and CGM experienced fewer acute events than those using only insulin pumps. Over a 5-year data collection period, there was a general rise in insulin pump utilization, increasing from 59% in 2017 to 66% in 2021. Hence, the insulin distribution sector has witnessed expansion.
End Use Insights
The hospitals and clinics segment is expected to witness the fastest CAGR of 8.42% during the forecast period. Hospitals play a crucial role in the insulin delivery industry, serving as hubs for diagnosing, treating, and managing diabetes. In hospital settings, various insulin delivery methods are employed to cater to patients' diverse needs. These include the use of insulin pumps, insulin pens, syringes, and other delivery devices. For instance, research conducted by the American Medical Association in February 2024 revealed that the utilization of home insulin pumps was considered safe for children hospitalized in non-intensive care units at a children's hospital, especially for hypoglycemia and hyperglycemia.
These pumps administer insulin continuously, replicating the natural pattern of insulin secretion observed in healthy pancreas.
The home care segment has captured the largest revenue share of 48.21% in 2023. Homecare plays a significant role in the insulin delivery industry, providing patients with the convenience of managing their diabetes at home. This sector encompasses various products and services tailored to support insulin administration, monitoring, and management outside clinical settings. Homecare companies offer a range of insulin delivery devices, such as insulin pens, syringes, pumps, and CGM systems. These devices enable patients to administer insulin doses accurately and regularly monitor their blood glucose levels without frequent visits to healthcare facilities.
Regional Insights
The North America insulin delivery devices market held the largest share of 40.73% in 2023 and is expected to maintain its dominance during the forecast period. Some key factors contributing to the region’s growth are the increasing geriatric population, the growing burden of diabetes due to lifestyle changes, the rising prevalence of obesity, and high treatment costs. Some of the key players, such as Abbott, Medtronic, and Dexcom, have their operational headquarters in North America, which is contributing to the market growth.
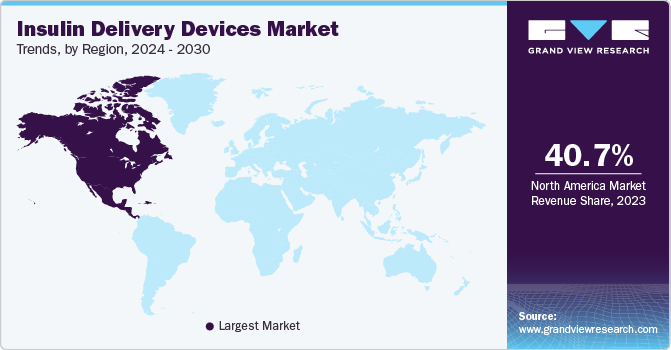
U.S. Insulin Delivery Devices Market Trends
The insulin delivery devices market in the U.S. held the largest market share in 2023 in the North America region. The high per capita income and increasing healthcare spending are among the key factors likely to drive market growth. The American Diabetes Association released the Economic Costs of Diabetes in the U.S. report in November 2023, highlighting the economic impact of diabetes in 2022. According to the report, the total cost of diabetes in 2022 was USD 412.9 billion, including USD 306.6 billion in direct medical costs and USD 106.3 billion in indirect costs. The report also mentioned that individuals diagnosed with diabetes now contribute to one-fourth of all healthcare expenses in the U.S.
Europe Insulin Delivery Devices Market Trends
The insulin delivery devices market in Europe held a significant market share in 2023. The increasing prevalence of diabetes and the rising awareness about diabetes management and control are some key factors expected to propel market growth over the forecast period. According to the International Diabetes Federation (IDF) report (2021), approximately 61 million people in Europe are diabetic, which accounts for around one in 11 adults. In addition, the Russian Federation, Germany, Spain, and Italy are the top countries in Europe with a high prevalence of diabetes.
The UK insulin delivery devices market is expected to exhibit the fastest CAGR during the forecast period owing to factors such as the rising burden of diabetes, well-established healthcare infrastructure, and growing healthcare expenditure on diabetes disease and its treatment. According to the National Health Service (NHS), as of December 2022, over 200,000 patients with type 1 diabetes have been using insulin delivery devices, which enable them to check their glucose levels conveniently and frequently. The NHS has made these devices available to patients with type 1 diabetes at a similar cost to flash monitors, and almost two-thirds of local NHS areas are currently providing this technology to patients. This has been a life-changing development for around eight in ten people with type 1 diabetes who now have access to this technology.
The insulin delivery devices market in France is closely linked to the country's diabetes landscape. Diabetes stands out as the most prevalent chronic condition, fully covered by France’s Statutory Health Insurance (SHI), with the number of covered patients doubling over the last decade. According to WHO, France has approximately one million diagnosed cases of diabetes. The prevalence of diabetes across all age groups in France is surging due to a rising obese population, unhealthy dietary habits, and sedentary lifestyles. In addition, around 0.26 million individuals are estimated to be diagnosed with type 1 diabetes.
The Germany insulin delivery devices market is experiencing significant growth, largely driven by the growing diabetes prevalence and country's aging population. Diabetes poses a significant health challenge for healthcare systems in Germany. The prevalence of type 1 & 2 diabetes in the adult population is high, with several patients remaining undiagnosed. The growing aging population and unhealthy lifestyle choices are expected to increase the prevalence of type 2 diabetes in the coming decade.
Asia Pacific Insulin Delivery Devices Market Trends
The Asia Pacific insulin delivery devices market is projected to grow at the fastest CAGR of 8.8% during the forecast period. The Asia Pacific market is majorly driven by China and Japan. China dominated the overall insulin delivery devices market in terms of revenue share. This can be attributed to the presence of major key players and the increasing prevalence of diabetes. In addition, technological advancements in insulin delivery devices, the increasing geriatric population, and the growing prevalence of lifestyle diseases, such as obesity, are some key factors anticipated to promote market growth. Furthermore, IDF has reported that a significant population of 90 million adults (aged 20-79) were living with diabetes in the IDF South-East Asia (SEA) Region in 2021. This number is expected to rise to 152 million by 2045. Similarly, in the IDF Western Pacific Region, around 206 million adults (aged 20-79) are living with diabetes in 2021, and this number is projected to increase to 260 million by 2045.
The insulin delivery devices market in China is expected to experience the fastest CAGR in the Asia Pacific region, driven by an increasing number of diabetes cases and rising costs associated with diabetes treatment and management. According to the Lancet Journal Forecast analysis, in Chinese adults aged between 20 to 79, the prevalence of diabetes is expected to rise from 8.2% to 9.7% between 2020 and 2030. Concurrently, diabetes-associated costs are projected to escalate from USD 250.2 billion to USD 460.4 billion, marking an annual growth rate of 6.32%. This increase will elevate diabetes-related expenses from 1.58% to 1.69% of China's GDP within the same timeframe, underscoring an acceleration in the economic impact of diabetes relative to the nation's economic expansion. In addition, the average economic burden per capita is set to grow from USD 231 to USD 414, at an annual rate of 6.02%. Notably, regions in Northeast and North China are identified as bearing significant disease and economic strain.
The Japan insulin delivery devices market is anticipated to expand at a CAGR of 8.9% from 2024 to 2030. As per the IDF data for 2021, the number of people with diabetes in Japan reached around 11 million. Type 1 diabetes is caused due to an immune system malfunction. However, a sedentary lifestyle, leading to inherent insulin resistance, causes type 2 diabetes. Japan has the greatest number of geriatric population, making them more susceptible to type 2 diabetes. With the growing geriatric population, the prevalence of diabetes is expected to rise. Hence, people are increasingly monitoring and managing their blood glucose levels to avoid serious health issues, such as kidney disorders and cardiovascular diseases.
The insulin delivery devices market in India is expected to grow owing to the increasing prevalence of diabetes disorder. The International Diabetes Federation (IDF) has projected that the global diabetic population will increase from 425 million in 2017 to around 629 million by 2045. Estimates from the Indian Council of Medical Research—India Diabetes (ICMR—INDIAB) suggest that approximately 62.4 million individuals in India are living with diabetes. In addition, it's worth noting that there is a decline in diabetes prevalence after the age of 65, which can be attributed to early-stage deaths resulting from comorbid conditions.
Latin America Insulin Delivery Devices Market Trends
The insulin delivery devices market in Latin America is experiencing growth due to the increasing incidence of Type 1 and Type 2 diabetes. Latin American countries have a high prevalence of diabetes, and Mexico is recognized for having many patients with diabetes as type-2 diabetes is becoming prevalent. The genetic predisposition to type-2 diabetes and the steadily increasing obesity incidence have been major contributors to the rise in type-2 diabetes cases over the past 40 years. Approximately 10% of people worldwide currently live with diabetes. The market growth is primarily driven by Brazil, Mexico, Argentina, and Colombia. Growing investments by market players in the region, proximity to North America, and free-trade agreements with major countries, such as the U.S., Canada, Japan, & several European countries, are among the factors anticipated to boost the market growth during the forecast period.
MEA Insulin Delivery Devices Market Trends
The insulin delivery devices market in MEA is expected to witness rapid growth due to the increased prevalence of diabetes and the rapidly aging population, as well as growing demand for home-based monitoring devices. According to the International Diabetes Federation (IDF), the Middle East accounted for the second-highest deaths due to diabetes among patients below 60 years. In addition, in 2021, IDF estimated that the current 24 million population with diabetes across the region is expected to increase by 134% by 2045. Despite significant factors driving the penetration of digital patient monitoring devices, stringent regulatory framework and lack of proper reimbursement policies are anticipated to restrain the market growth. In the IDF MENA region, it is estimated that around 73 million adults between the ages of 20 to 79 are currently living with diabetes, and this figure is expected to rise to 95 million by 2030. In addition, about 48 million adults in the region have IGT, which increases their risk of developing type 2 diabetes. Diabetes has caused around 796,000 deaths in the IDF MENA region this year. The healthcare costs associated with diabetes in 2021 amounted to approximately USD 33 billion.
Key Insulin Delivery Devices Company Insights
The insulin delivery devices market is a dynamic and rapidly evolving segment within the medical device industry, primarily driven by the increasing prevalence of diabetes globally. This market includes a range of devices designed to administer insulin to diabetic patients, aiming to maintain their blood glucose levels. As diabetes management shifts towards more patient-friendly, efficient, and less invasive methods, the demand for innovative insulin delivery devices has surged. The insulin delivery devices market is highly competitive, with companies continuously innovating and expanding their product lines to meet the diverse needs of diabetes patients. Market share is influenced by factors such as product innovation, ease of use, accessibility, and insurance coverage. Major players such as Novo Nordisk, Sanofi, and Eli Lilly dominate the market due to their extensive product ranges, global presence, and strong brand recognition. However, smaller companies and startups are also making significant inroads by focusing on niche segments, such as wearable insulin delivery devices and smart insulin pens, challenging the dominance of established players.
Key Insulin Delivery Devices Companies:
The following are the leading companies in the insulin delivery devices market. These companies collectively hold the largest market share and dictate industry trends.
- Novo Nordisk A/S
- Sanofi
- Eli Lilly and Company
- Ypsomed AG
- Medtronic
- Insulet Corporation
- B. Braun SE
- Owen Mumford Ltd.
- Tandem Diabetes Care, Inc.
- BD (Becton, Dickinson, and Company)
Recent Developments
-
In August 2023, Novo Nordisk A/S and Inversago Pharma have reached an agreement wherein Novo Nordisk will acquire Inversago for a sum that could reach up to 1.075 billion US dollars in cash, contingent upon the fulfillment of specific development and commercial benchmarks. Based in Montreal, Inversago Pharma is a privately held company specializing in the development of therapies targeting the CB1 receptor aimed at potentially treating obesity, diabetes, and related metabolic disorder complications.
“The acquisition of Inversago Pharma will further strengthen our clinical development pipeline in obesity and related disorders.”
-Martin Holst Lange, executive vice president for Development at Novo Nordisk.
-
In March 2023, Diabeloop, a forefront company in Automated Insulin Delivery technology, announced a partnership with the renowned healthcare giant, Novo Nordisk. This collaboration focuses on the integration of Diabeloop's advanced self-learning algorithm, DBL-4pen, specifically designed for Multiple Daily Injections (MDI) therapy, with Novo Nordisk's innovative, connected, and reusable insulin pens, namely NovoPen 6 and NovoPen Echo Plus. To assess the effectiveness and clinical advantages of this integrated solution, Diabeloop is gearing up to conduct a specialized study aimed at individuals diagnosed with Type 2 diabetes.
-
Sanofi announced a significant reduction in the list price for its widely used insulin product, Lantus (insulin glargine injection) 100 Units/mL, in the U.S., slashing it by 78%. In addition, the company introduced a cap of $35 on out-of-pocket expenses for all commercially insured patients needing Lantus, highlighting its ongoing dedication to making medications more affordable. These actions, effective from January 1, 2024, build upon earlier measures from June 2022 aimed at reducing the cost of diabetes medications. These included releasing a non-branded version of Lantus at 60% below its list price and implementing a $35 maximum on the out-of-pocket costs for insulin for individuals without insurance. Thanks to these comprehensive efforts, Sanofi has ensured that patients will not spend over $35 for a month's supply of Lantus. In a further step, Sanofi also announced a 70% reduction in the list price of its short-acting insulin, Apidra (insulin glulisine injection) 100 Units/mL.
-
In November 2022, Novo Nordisk and Abbott announced their partnership aiming enhanced data integration capabilities for individuals with diabetes. This development leverages smart technology by making Novo Nordisk's connected insulin pens, specifically the NovoPen 6 and NovoPen Echo Plus, compatible with the Abbott FreeStyle LibreLink application. This compatibility facilitates a centralized view of insulin dosage and glucose levels, streamlining patient monitoring and management. Users can effortlessly sync their insulin dosing information by simply tapping their pen on a smartphone, enabling a cohesive display of insulin and glucose data. This integration aims to aid patients in analyzing how insulin dosage adjustments and timing influence their glucose trends, thereby optimizing diabetes management.
“People living with diabetes can make up to 180 additional health-related decisions a day compared to people without diabetes - the constant multi-tasking can be emotionally and physically draining. I hope that bringing glucose and insulin data together in one place will make some of these decisions a little easier, giving people living with diabetes in the UK more time and energy back for day-to-day life.”
-Pinder Sahota, general manager at Novo Nordisk UK.
Insulin Delivery Devices Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 17.80 billion
Revenue forecast in 2030
USD 28.06 billion
Growth rate
CAGR of 7.89% from 2024 to 2030
Base year for estimation
2023
Actual period
2018 - 2022
Forecast period
2024 - 2030
Quantitative units
Revenue in USD million and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, end use
Regional scope
North America, Europe, Asia Pacific, Latin America, MEA
Country scope
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; Thailand; South Korea; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Novo Nordisk A/S; Sanofi; Eli Lilly and Company; Ypsomed AG; Medtronic; Insulet Corporation; B. Braun SE; Owen Mumford Ltd.; Tandem Diabetes Care, Inc.; BD (Becton, Dickinson and Company)
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
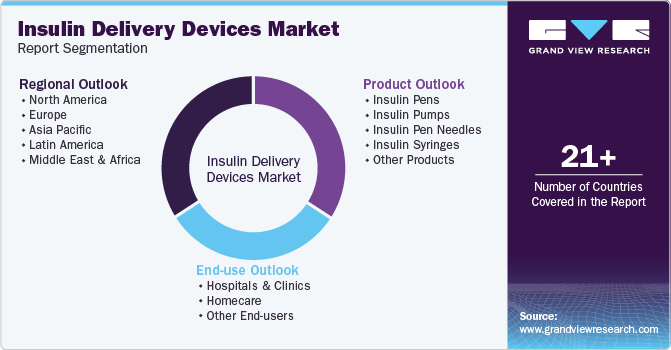
Global Insulin Delivery Devices Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global insulin delivery devices market report based on product, end use, and region:
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Insulin Pens
-
Reusable Insulin Pens
-
Disposable Insulin Pens
-
-
Insulin Pumps
-
Patch Pumps
-
Tethered Pumps
-
-
Insulin Pen Needles
-
Standard Pen Needles
-
Safety Pen Needles
-
-
Insulin Syringes
-
Other Products
-
-
End Use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals & Clinics
-
Homecare
-
Other End Users
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
Thailand
-
South Korea
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global insulin delivery devices market size was estimated at USD 16.43 billion in 2023 and is expected to reach USD 17.80 billion in 2024.
b. The global insulin delivery devices market is expected to grow at a compound annual growth rate of 7.89% from 2024 to 2030 to reach USD 28.06 Billion by 2030.
b. The insulin pens segment dominated the insulin delivery devices market with the highest share in 2023. This is attributable to the increasing prevalence of diabetes.
b. Some key players operating in the insulin delivery devices market include Medtronic, Novo Nordisk A/S, Sanofi, Eli Lilly and Company, Ypsomed AG, Insulet Corporation, B. Braun SE, Owen Mumford Ltd., BD (Becton, Dickinson and Company), and Tandem Diabetes Care, Inc.
b. Key factors driving the insulin delivery devices market include the increasing prevalence of diabetes worldwide, advancements in insulin administration technology, growing awareness among patients about the benefits of automated insulin delivery devices over traditional syringe methods, and the convenience and ease of use offered by them.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










