- Home
- »
- Pharmaceuticals
- »
-
Blastic Plasmacytoid Dendritic Cell Neoplasm Market Report, 2030GVR Report cover
![Blastic Plasmacytoid Dendritic Cell Neoplasm Market Size, Share & Trends Report]()
Blastic Plasmacytoid Dendritic Cell Neoplasm Market (2024 - 2030) Size, Share & Trends Analysis Report By Treatment (Chemotherapy, Immunotherapy, Stem Cell Transplantation), By End Use (Hospitals, Specialty Clinics), And Segment Forecasts
- Report ID: GVR-4-68040-427-8
- Number of Report Pages: 120
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2024 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Market Size & Trends
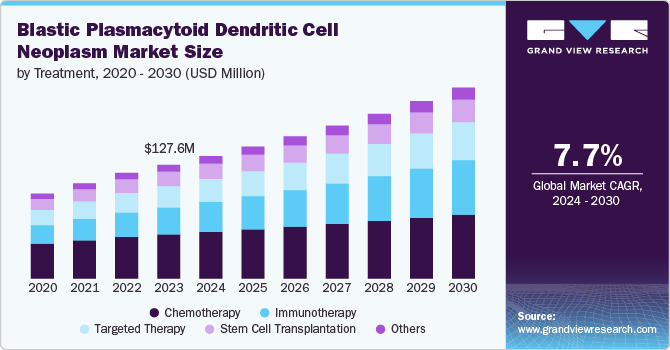
The global blastic plasmacytoid dendritic cell neoplasm market size was estimated at USD 127.65 million in 2023 and is projected to grow at a CAGR of 7.7% from 2024 to 2030. The market is driven by several critical factors, including increasing incidence rates of BPDCN, advancements in treatment options, growing awareness and diagnosis of rare hematological malignancies, and supportive regulatory frameworks. These drivers collectively contribute to the evolving landscape of blastic plasmacytoid dendritic cell neoplasm (BPDCN) management and treatment. According to the National Centre for Biotechnology Information, they recognize BPDCN as a unique disease that led to evolving treatment strategies, moving from traditional chemotherapy and stem cell transplants to promising CD123-targeted immunotherapies like SL-401 and CAR T-cells.
The increasing incidence of BPDCN is a primary driver for this market’s growth. Rising incidence highlights the critical need for effective treatment options, as BPDCN is diagnosed late due to its rarity and aggressive nature. As awareness of BPDCN increases among healthcare professionals, timely diagnosis and intervention become more feasible, ultimately driving demand for specialized therapies and enhancing the overall market for BPDCN treatments.
The growing demand for effective therapies is another significant driver in the BPDCN market. Typically, individuals diagnosed with BPDCN have a bleak outlook, with average life expectancy falling below one year if they do not receive any treatment. The recent approval of therapies such as tagraxofusp generated considerable interest among clinicians and patients alike, as it demonstrated efficacy in achieving complete remission in clinical trials. The need for personalized medicine further fuels this demand for innovative treatments that cater to the unique characteristics of BPDCN, encouraging pharmaceutical companies to invest in developing targeted therapies.
Market Concentration & Characteristics
Innovation in the blastic plasmacytoid dendritic cell neoplasm market is high, driven by advancements in targeted therapies and immunotherapies. The ongoing research into additional immunotherapies and combination treatments reflects a robust pipeline aimed at improving patient outcomes. The innovation in this segment is driven by the urgent need for effective therapies for this rare malignancy, with clinical trials expanding to evaluate new drug combinations and treatment regimens. The focus on innovative treatments is expected to grow as more data emerges from ongoing studies, indicating a high level of innovation in the market.
The level of merger and acquisition (M&A) activities in the BPDCN market is medium as companies seek to enhance their portfolios with innovative therapies. These M&A activities are driven by the need to acquire novel technologies or drugs to address unmet medical needs in the BPDCN space. As the market evolves, further consolidation may occur, enabling companies to leverage synergies and expand their therapeutic capabilities, which could increase M&A activities in the coming years.
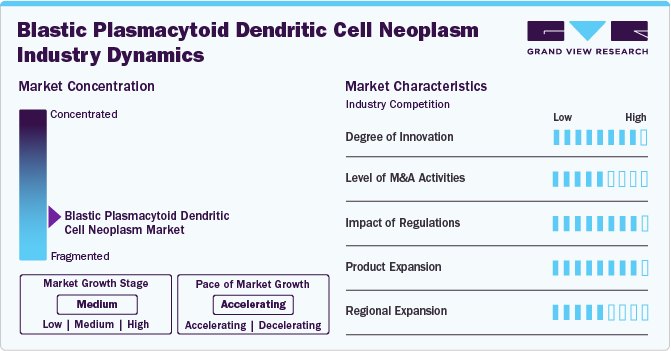
Regulatory impacts on the BPDCN market are significant, with high scrutiny and support from agencies like the FDA and EMA. Regulatory support can accelerate patients' access to new therapies but requires companies to navigate complex compliance landscapes. This support is crucial for driving market growth, as it facilitates faster access to treatments for patients and incentivizes pharmaceutical companies to invest in research and development for BPDCN. The proactive regulatory framework is expected to continue fostering an environment conducive to introducing new therapies.
In the BPDCN market, product expansion is at a high level, driven by the introduction of innovative therapies that target this rare malignancy. Ongoing clinical trials explore new agents, such as UCART123 and other immunotherapies, to enhance treatment efficacy and broaden the therapeutic landscape. The focus on developing combination therapies further illustrates the commitment to expanding treatment options for BPDCN, as evidenced by studies investigating the synergistic effects of existing chemotherapy regimens with novel agents.
Regional expansion activities in the BPDCN market are at a medium level as companies and healthcare providers work to improve access to treatments in underserved areas. Efforts are being made to establish specialized treatment centers and clinics focusing on rare hematological malignancies, particularly in regions with limited healthcare resources. Initiatives to enhance awareness and training among healthcare professionals are being implemented to facilitate earlier diagnosis and treatment of BPDCN. Additionally, pharmaceutical companies actively seek to enter new markets, especially in emerging economies, to ensure that innovative therapies reach a broader patient population.
Treatment Insights
Chemotherapy held the largest revenue share of 38.7% in 2023, primarily due to its established role as a frontline treatment option. According to the NCBI, BPDCN is known to be sensitive to chemotherapy when using regimens typically designed for acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML). This effectiveness drives the continued reliance on chemotherapy despite the disease's aggressive nature and the challenges associated with treatment resistance. Additionally, the ongoing development of combination therapies and the integration of chemotherapy with newer treatments, such as tagraxofusp, enhance the overall treatment landscape for BPDCN.
The immunotherapy segment emerged as the fastest-growing category in the market, driven by the increasing recognition of targeted therapies that improve patient outcomes. Ongoing clinical trials explore combination therapies that integrate immunotherapy with existing treatment modalities, enhancing the potential for improved survival rates. The growing emphasis on research and development in immunotherapy reflects a broader trend in oncology, where specialized treatments are increasingly tailored to the unique characteristics of rare malignancies, including BPDCN.
End Use Insights
Hospitals held the largest revenue share of 57.5% in 2023, primarily due to the increasing incidence of BPDCN and the need for specialized treatment facilities. Moreover, hospitals are equipped with advanced diagnostic equipment and treatment options, including chemotherapy and stem cell transplantation, which are critical for managing this rare hematological malignancy. The rising awareness of BPDCN among healthcare professionals and patients led to more hospital referrals for comprehensive care. Instances such as clinical trials conducted within hospital settings and collaborations between hospitals and pharmaceutical companies further drive revenue growth in this segment.
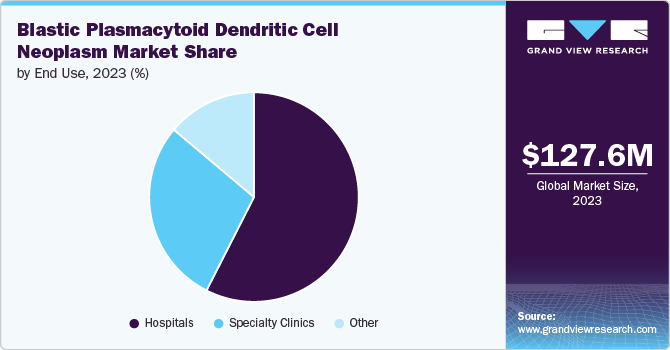
The specialty clinics segment emerged as the fastest-growing CAGR of 9.1% in the blastic plasmacytoid dendritic cell neoplasm market due to several key drivers, including the increasing prevalence of hematological malignancies, advancements in targeted therapies, and a growing focus on personalized medicine. Specialty clinics offer tailored treatment plans and access to cutting-edge clinical trials, which attract patients seeking specialized care. Clinics focusing on rare blood cancers are expanding their services and capabilities to provide comprehensive management of blastic plasmacytoid dendritic cell neoplasm, enhancing patient outcomes and driving market growth.
Regional Insights
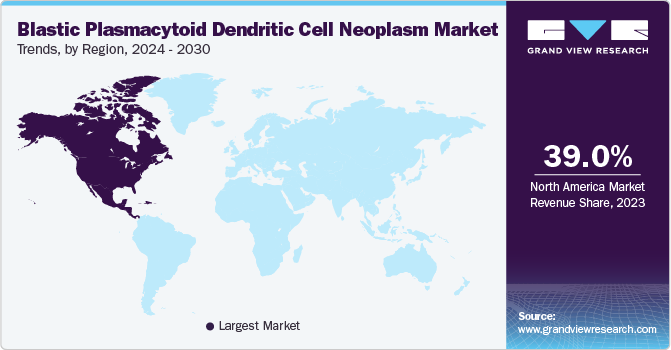
North America blastic plasmacytoid dendritic cell neoplasm market is the largest revenue contributor, with over 39.0% share in 2023. A growing recognition of this rare hematological malignancy characterizes North America's blastic plasmacytoid dendritic cell neoplasm market. Increased awareness initiatives among healthcare professionals are crucial, as timely diagnosis significantly impacts patient outcomes. Approving therapies further stimulated interest in BPDCN treatment options. Additionally, the collaboration between academic institutions and pharmaceutical companies fosters research into innovative therapies, which is essential given the aggressive nature of BPDCN and the limited treatment options available.
U.S. Blastic Plasmacytoid Dendritic Cell Neoplasm Market Trends
Blastic plasmacytoid Dendritic Cell Neoplasm market in the U.S. is seeing significant advancements in diagnosis and treatment. According to NCBI, Blastic plasmacytoid dendritic cell neoplasia (BPDCN) is an uncommon hematologic disorder with a poor prognosis. Its incidence in the United States is reported at 0.04 cases per 100,000 people, accounting for less than 1.0% of all hematologic cancers. Furthermore, establishing specialized treatment centers focused on rare malignancies provides patients with better access to novel therapies.
Europe Blastic Plasmacytoid Dendritic Cell Neoplasm Market Trends
The European blastic plasmacytoid dendritic cell neoplasm market is experiencing a notable trend towards increased research and development of targeted therapies. The European Medicines Agency (EMA) is actively involved in approving new treatments, including targeted therapies and immunotherapies, which have demonstrated encouraging results in enhancing patient outcomes. Recent studies indicate that the estimated annual incidence of BPDCN in the US and European combined populations ranges from 1,000 to 1,400 cases.
The UK's blastic plasmacytoid dendritic cell neoplasm market is marked by increasing efforts to enhance diagnostic capabilities and treatment accessibility. The National Health Service (NHS) prioritized the development of specialized hematology centers, which are essential for the timely diagnosis and management of BPDCN. Initiatives focused on training healthcare professionals to recognize this rare disease. Furthermore, the UK is witnessing the launch of clinical trials evaluating novel therapies, which are critical for expanding treatment options and improving patient outcomes.
France blastic plasmacytoid dendritic cell neoplasm market is witnessing significant advancements in treatment accessibility and patient care. The country's healthcare system integrated tagraxofusp into clinical practice. The French government's commitment to rare diseases led to increased funding for research and the establishment of specialized treatment centers, enhancing awareness and improving management strategies for BPDCN.
Asia Pacific Blastic Plasmacytoid Dendritic Cell Neoplasm Market Trends
The blastic plasmacytoid dendritic cell neoplasm market in APAC increasingly emphasizes expanding treatment options and improving patient outcomes. The market is driven by increasing awareness of rare hematological malignancies and advancements in diagnostic techniques. Innovative therapies, such as targeted treatments and immunotherapies, are being developed to improve patient outcomes.
Japan blastic plasmacytoid dendritic cell neoplasm market is focusing on R&D of targeted therapies. The country saw a rise in clinical trials evaluating novel agents for BPDCN, such as antibody-drug conjugates and immunotherapies. The Japanese government has been actively supporting research initiatives and providing funding for the development of innovative therapies. For instance, in August 2023, the Japanese Ministry of Health, Labor, and Welfare awarded orphan drug status to tagraxofusp-erzs (Elzonris) for the treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN), acknowledging its potential advantages for patients with few available treatment options.
The blastic plasmacytoid dendritic cell neoplasm market in China is witnessing growth due to its large patient population and increasing healthcare expenditure. The government actively promotes research into rare diseases through funding initiatives and policy support. According to a report published by the National Centre for Biotechnology Information (NCBI) in June 2024, in Chinese patients, BPDCN cells show a 2.52 male-to-female ratio, a median onset age of 50 for adults and 10 for children, with distinct immune phenotypes.
Latin America Blastic Plasmacytoid Dendritic Cell Neoplasm Market Trends
The blastic plasmacytoid dendritic cell neoplasm market in Latin America is witnessing a growing emphasis on improving diagnostic capabilities and increasing awareness among healthcare professionals. The region saw a rise in specialized cancer treatment centers equipped with advanced diagnostic tools and trained personnel to identify BPDCN accurately. Furthermore, the increasing awareness about the disease and its diagnosis is leading to a rise in the number of patients seeking treatment, contributing to the market's growth.
The blastic plasmacytoid dendritic cell neoplasm market in Brazil focuses on expanding treatment options and improving patient outcomes. The country witnessed the launch of new therapies, such as tagraxofusp, which received regulatory approval for treating BPDCN. This development significantly impacted the market, providing patients with an additional treatment option. Furthermore, the Brazilian government was actively investing in healthcare infrastructure, establishing specialized hematology centers, and implementing multidisciplinary approaches to BPDCN management. These efforts contributed to increased awareness and better patient outcomes, driving the growth of Brazil's BPDCN market.
Middle East & Africa Blastic Plasmacytoid Dendritic Cell Neoplasm Market Trends
The blastic plasmacytoid dendritic cell neoplasm market in the Middle East and Africa is characterized by a growing awareness of rare hematological malignancies, leading to increased R&D efforts. Innovative treatment options, including targeted therapies and immunotherapies, are being explored to improve patient outcomes. The region witnessed collaborations between pharmaceutical companies and local healthcare providers to enhance access to novel treatments. Additionally, regulatory bodies are becoming more proactive in approving new therapies, which is expected to drive market growth.
The blastic plasmacytoid dendritic cell neoplasm market in Saudi Arabia is influenced by advancements in cancer treatment protocols and increased clinical trials focusing on rare diseases. The Kingdom’s Vision 2030 initiative emphasizes improving healthcare infrastructure and fostering innovation in medical research. Furthermore, the approval and launch of targeted therapies, such as pivekimab sunrise, are anticipated to transform the treatment landscape for BPDCN in the country. Establishing specialized oncology centers improved diagnosis and treatment capabilities for patients suffering from this rare neoplasm.
Key Blastic Plasmacytoid Dendritic Cell Neoplasm Company Insights
The blastic plasmacytoid dendritic cell neoplasm (BPDCN) market is characterized by a few key players that dominate the landscape, focusing on innovative therapies and treatment options. The market share is primarily held by companies that developed targeted therapies and novel agents specifically for BPDCN, reflecting a trend toward personalized medicine in oncology. The overall market is expected to grow as awareness of BPDCN increases and more treatment options become available.
Key Blastic Plasmacytoid Dendritic Cell Neoplasm Companies:
The following are the leading companies in the blastic plasmacytoid dendritic cell neoplasm market. These companies collectively hold the largest market share and dictate industry trends.
- AbbVie Inc.
- ImmunoGen, Inc.
- Mustang Bio
- Genentech, Inc.
- Stemline Therapeutics, Inc.
- Jazz Pharmaceuticals, Inc.
- Cellex Patient Treatment GmbH
- Xencor
- Resverlogix
Recent Developments
-
In November 2023, AbbVie's acquisition of ImmunoGen, including ELAHERE for ovarian cancer, expanded its solid tumor portfolio and included pivekimab sunrise, an investigational ADC targeting blastic plasmacytoid dendritic cell neoplasm, a rare blood cancer.
-
In June 2023, ImmunoGen presented updated results from the CADENZA trial evaluating pivekimab sunrise in blastic plasmacytoid dendritic cell neoplasm at the EHA 2023 Congress. The results demonstrated anti-tumor activity and favorable tolerability in frontline and relapsed/refractory patients.
Blastic Plasmacytoid Dendritic Cell Neoplasm Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 137.45 million
Revenue forecast in 2030
USD 214.27 million
Growth rate
CAGR of 7.7% from 2024 to 2030
Historical data
2018 - 2023
Forecast period
2024 - 2030
Quantitative units
Revenue in USD million and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Treatment, end use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; and MEA
Country scope
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Sweden; Denmark; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Argentina; Saudi Arabia; South Africa; UAE; Kuwait
Key companies profiled
AbbVie Inc.; ImmunoGen Inc.; Mustang Bio; Genentech, Inc.; Stemline Therapeutics, Inc.; Jazz Pharmaceuticals, Inc.; Cellex Patient Treatment GmbH; Xencor; Resverlogix
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Blastic Plasmacytoid Dendritic Cell Neoplasm Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global blastic plasmacytoid dendritic cell neoplasm market report based on treatment, end use and region.
-
Blastic Plasmacytoid Dendritic Cell Neoplasm Treatment Outlook (Revenue, USD Million, 2018 - 2030)
-
Chemotherapy
-
Immunotherapy
-
Stem Cell Transplantation
-
Targeted Therapy
-
Other
-
-
Blastic Plasmacytoid Dendritic Cell Neoplasm End Use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Specialty Clinics
-
Other
-
-
Blastic Plasmacytoid Dendritic Cell Neoplasm Market Report Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global blastic plasmacytoid dendritic cell neoplasm market size was valued at USD 127.65 million in 2023 and is projected to reach USD 137.45 million by 2024.
b. The global blastic plasmacytoid dendritic cell neoplasm market is projected to grow at a compounded annual growth rate (CAGR) of 7.68% from 2024 to 2030 to reach USD 214.27 million by 2030.
b. Chemotherapy held the largest revenue share of 38.7% in 2023, primarily due to its established role as a frontline treatment option.
b. Some of the key players operating in this market include AbbVie Inc., ImmunoGen, Inc., Mustang Bio, Genentech, Inc., Stemline Therapeutics, Inc., Jazz Pharmaceuticals, Inc., Cellex Patient Treatment GmbH, Xencor, Resverlogix.
b. The market is driven by several critical factors, including increasing incidence rates of BPDCN, advancements in treatment options, growing awareness and diagnosis of rare hematological malignancies, and supportive regulatory frameworks.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










