Europe Regulatory Affairs Outsourcing Market Size & Outlook
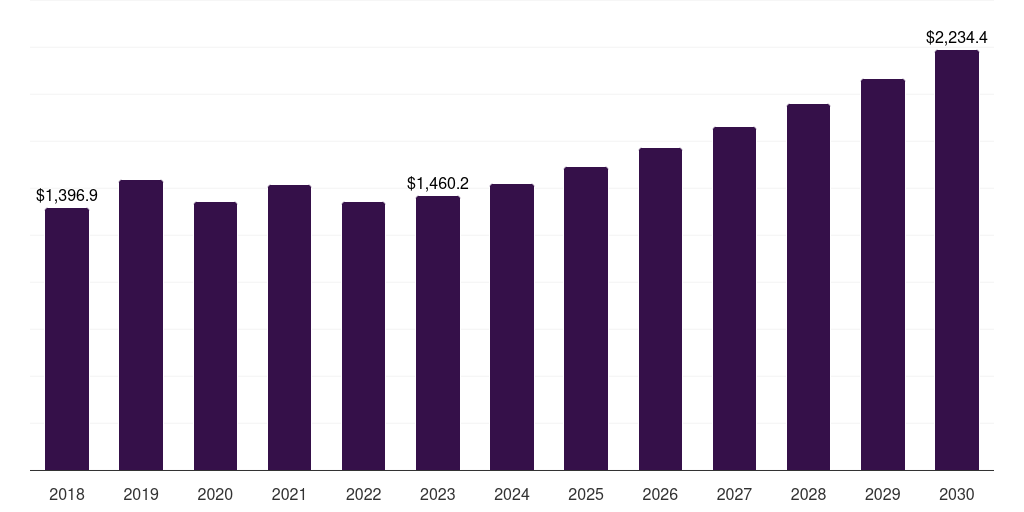
Europe regulatory affairs outsourcing market, 2018-2030 (US$M)
Related Markets
Europe regulatory affairs outsourcing market highlights
- The Europe regulatory affairs outsourcing market generated a revenue of USD 1,460.2 million in 2023.
- The market is expected to grow at a CAGR of 6.3% from 2024 to 2030.
- In terms of segment, product registration & clinical trial applications was the largest revenue generating service in 2023.
- Legal representation is the most lucrative service segment registering the fastest growth during the forecast period.
- Country-wise, UK is expected to register the highest CAGR from 2024 to 2030.
Europe data book summary
| Market revenue in 2023 | USD 1,460.2 million |
| Market revenue in 2030 | USD 2,234.4 million |
| Growth rate | 6.3% (CAGR from 2023 to 2030) |
| Largest segment | Product registration & clinical trial applications |
| Fastest growing segment | Legal representation |
| Historical data covered | 2018 - 2022 |
| Base year for estimation | 2023 |
| Forecast period covered | 2024 - 2030 |
| Quantitative units | Revenue in USD million |
| Market segmentation | Regulatory consulting, Legal representation, Regulatory writing & publishing, Product registration & clinical trial applications, Regulatory Submissions, Regulatory Operations, Other services |
| Key market players worldwide | Genpact Ltd, Criterium Inc., Promedica International, Wuxi AppTec Co Ltd, Medpace Holdings Inc, Charles River Laboratories International Inc, Icon PLC, Labcorp Drug Development, FREYR Battery Inc, Cencora Inc, Pharmexon, QVigilance, BlueReg Group, ZEINCRO Group |
Other key industry trends
- In terms of revenue, Europe region accounted for 22.0% of the global regulatory affairs outsourcing market in 2023.
- Globally, Asia Pacific is projected to lead the regional market in terms of revenue in 2030.
- Asia Pacific is the fastest growing regional market and is projected to reach USD 5,028.6 million by 2030.
No credit card required*
Horizon in a snapshot
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more
Regulatory Affairs Outsourcing Market Scope
Regulatory Affairs Outsourcing Market Companies
| Name | Profile | # Employees | HQ | Website |
|---|
Europe regulatory affairs outsourcing market outlook
The databook is designed to serve as a comprehensive guide to navigating this sector. The databook focuses on market statistics denoted in the form of revenue and y-o-y growth and CAGR across the globe and regions. A detailed competitive and opportunity analyses related to regulatory affairs outsourcing market will help companies and investors design strategic landscapes.
Product registration & clinical trial applications was the largest segment with a revenue share of 25.96% in 2023. Horizon Databook has segmented the Europe regulatory affairs outsourcing market based on regulatory consulting, legal representation, regulatory writing & publishing, product registration & clinical trial applications, regulatory submissions, regulatory operations, other services covering the revenue growth of each sub-segment from 2018 to 2030.
Europe is a decentralized pharmaceutical market. Entry in the European market requires players to possess extensive knowledge about different regulatory procedures in various member states of the European Union.
Small- and mid-size biopharmaceutical companies that do not have a regulatory affairs department usually hire a regulatory consultant or a legal representative to assist them through various stages of regulatory approval processes required for commercializing their product in Europe.
The European Medicine Agency (EMA), Europe’s regulatory agency covers 25.0% market of worldwide pharmaceutical sales and this is one of the leading drivers for outsourcing trend related to regulatory affairs in the life sciences sector as higher number of drugs for regulatory approval would mean greater demand for regulatory services by life sciences companies in the region.
Reasons to subscribe to Europe regulatory affairs outsourcing market databook:
-
Access to comprehensive data: Horizon Databook provides over 1 million market statistics and 20,000+ reports, offering extensive coverage across various industries and regions.
-
Informed decision making: Subscribers gain insights into market trends, customer preferences, and competitor strategies, empowering informed business decisions.
-
Cost-Effective solution: It's recognized as the world's most cost-effective market research database, offering high ROI through its vast repository of data and reports.
-
Customizable reports: Tailored reports and analytics allow companies to drill down into specific markets, demographics, or product segments, adapting to unique business needs.
-
Strategic advantage: By staying updated with the latest market intelligence, companies can stay ahead of competitors, anticipate industry shifts, and capitalize on emerging opportunities.
Target buyers of Europe regulatory affairs outsourcing market databook
-
Our clientele includes a mix of regulatory affairs outsourcing market companies, investment firms, advisory firms & academic institutions.
-
30% of our revenue is generated working with investment firms and helping them identify viable opportunity areas.
-
Approximately 65% of our revenue is generated working with competitive intelligence & market intelligence teams of market participants (manufacturers, service providers, etc.).
-
The rest of the revenue is generated working with academic and research not-for-profit institutes. We do our bit of pro-bono by working with these institutions at subsidized rates.
Horizon Databook provides a detailed overview of continent-level data and insights on the Europe regulatory affairs outsourcing market , including forecasts for subscribers. This continent databook contains high-level insights into Europe regulatory affairs outsourcing market from 2018 to 2030, including revenue numbers, major trends, and company profiles.
Partial client list
Europe regulatory affairs outsourcing market size, by country, 2018-2030 (US$M)
Europe Regulatory Affairs Outsourcing Market Outlook Share, 2023 & 2030 (US$M)
Related industry reports
Related regional statistics
Sign up - it's easy, and free!
Sign up and get instant basic access to databook, upgrade
when ready, or enjoy our
free plan indefinitely.
Included in Horizon account
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more