Saudi Arabia Preclinical Cro Market Size & Outlook
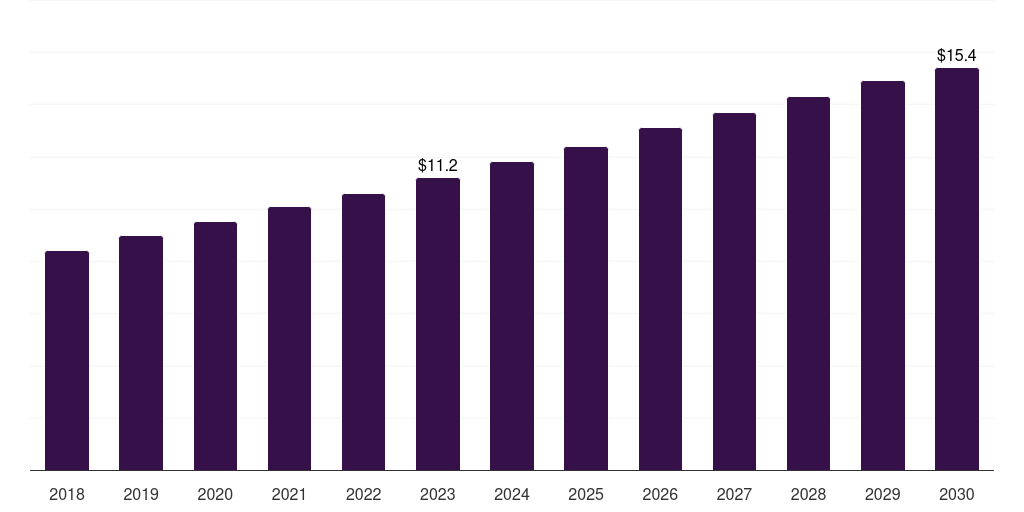
Saudi Arabia preclinical cro market, 2018-2030 (US$M)
Related Markets
Saudi Arabia preclinical cro market highlights
- The Saudi Arabia preclinical cro market generated a revenue of USD 11.2 million in 2023 and is expected to reach USD 15.5 million by 2030.
- The Saudi Arabia market is expected to grow at a CAGR of 4.7% from 2024 to 2030.
- In terms of segment, toxicology testing was the largest revenue generating service in 2023.
- Bioanalysis and DMPK studies is the most lucrative service segment registering the fastest growth during the forecast period.
Preclinical cro market data book summary
| Market revenue in 2023 | USD 11.2 million |
| Market revenue in 2030 | USD 15.5 million |
| Growth rate | 4.7% (CAGR from 2023 to 2030) |
| Largest segment | Toxicology testing |
| Fastest growing segment | Bioanalysis and DMPK studies |
| Historical data | 2018 - 2022 |
| Base year | 2023 |
| Forecast period | 2024 - 2030 |
| Quantitative units | Revenue in USD million |
| Market segmentation | Bioanalysis and DMPK studies, Toxicology Testing, Compound Management, Chemistry, Safety Pharmacology |
| Key market players worldwide | Eurofins Scientific SE, Icon PLC, Wuxi AppTec Co Ltd, Medpace Holdings Inc, Charles River Laboratories International Inc, Thermo Fisher Scientific Inc, Intertek Group PLC, Labcorp Holdings Inc, Crown Bioscience, SGA |
Other key industry trends
- In terms of revenue, Saudi Arabia accounted for 0.2% of the global preclinical cro market in 2023.
- Country-wise, U.S. is expected to lead the global market in terms of revenue in 2030.
- In Middle East & Africa, South Africa preclinical cro market is projected to lead the regional market in terms of revenue in 2030.
- South Africa is the fastest growing regional market in Middle East & Africa and is projected to reach USD 30.5 million by 2030.
Toxicology testing was the largest segment with a revenue share of 22.32% in 2023. Horizon Databook has segmented the Saudi Arabia preclinical cro market based on bioanalysis and dmpk studies, toxicology testing, compound management, chemistry, safety pharmacology covering the revenue growth of each sub-segment from 2018 to 2030.
In 2023, Saudi Arabia held a share of 20.37% in the MEA market. Demand in the country can be attributed to the increasing demand for biosimilar & biological therapies, rising investments in R&D, and technological advancements, such as Artificial Intelligence (AI) & Big data.
The healthcare industry in Saudi Arabia is expected to gain substantial benefits from the integration of AI. The country is implementing significant AI-based measures to enhance its technological capabilities.
For instance, in May 2021, IQVIA, a leading health information technology, and clinical research company from the U.S., announced a cooperation with the Saudi Data and Artificial Intelligence Authority (SDAIA).
No credit card required*
Horizon in a snapshot
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more
Preclinical CRO Market Scope
Preclinical CRO Market Companies
| Name | Profile | # Employees | HQ | Website |
|---|
Saudi Arabia preclinical cro market size, by service, 2018-2030 (US$M)
Saudi Arabia Preclinical CRO Market Outlook Share, 2023 & 2030 (US$M)
Related industry reports
Related regional statistics
Sign up - it's easy, and free!
Sign up and get instant basic access to databook, upgrade
when ready, or enjoy our
free plan indefinitely.
Included in Horizon account
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more