North America Preclinical Cro Market Size & Outlook
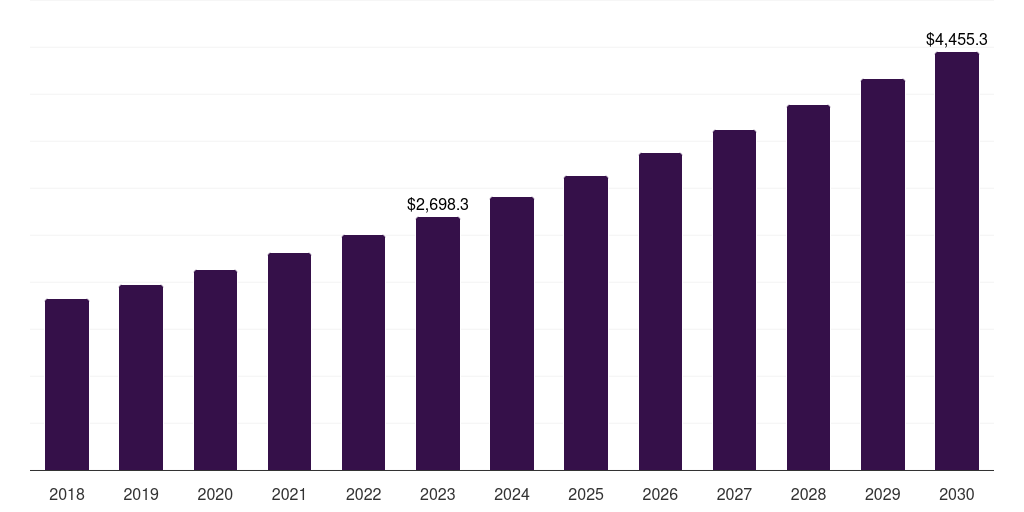
North America preclinical cro market, 2018-2030 (US$M)
Related Markets
North America preclinical cro market highlights
- The North America preclinical cro market generated a revenue of USD 2,698.2 million in 2023.
- The market is expected to grow at a CAGR of 7.4% from 2024 to 2030.
- In terms of segment, toxicology testing was the largest revenue generating service in 2023.
- Bioanalysis and DMPK studies is the most lucrative service segment registering the fastest growth during the forecast period.
- Country-wise, U.S. is expected to register the highest CAGR from 2024 to 2030.
North America data book summary
| Market revenue in 2023 | USD 2,698.2 million |
| Market revenue in 2030 | USD 4,455.2 million |
| Growth rate | 7.4% (CAGR from 2023 to 2030) |
| Largest segment | Toxicology testing |
| Fastest growing segment | Bioanalysis and DMPK studies |
| Historical data covered | 2018 - 2022 |
| Base year for estimation | 2023 |
| Forecast period covered | 2024 - 2030 |
| Quantitative units | Revenue in USD million |
| Market segmentation | Bioanalysis and DMPK studies, Toxicology Testing, Compound Management, Chemistry, Safety Pharmacology |
| Key market players worldwide | Eurofins Scientific SE, Icon PLC, Wuxi AppTec Co Ltd, Medpace Holdings Inc, Charles River Laboratories International Inc, Thermo Fisher Scientific Inc, Intertek Group PLC, Labcorp Holdings Inc, Crown Bioscience, SGA |
Other key industry trends
- In terms of revenue, North America region accounted for 47.2% of the global preclinical cro market in 2023.
- Globally, North America is projected to lead the regional market in terms of revenue in 2030.
- Asia Pacific is the fastest growing regional market and is projected to reach USD 1,865.2 million by 2030.
Toxicology testing was the largest segment with a revenue share of 26.15% in 2023. Horizon Databook has segmented the North America preclinical cro market based on bioanalysis and dmpk studies, toxicology testing, compound management, chemistry, safety pharmacology covering the revenue growth of each sub-segment from 2018 to 2030.
North America accounts for the largest share in the global preclinical trials outsourcing market owing to the presence of established CROs specializing in early drug discovery, such as Charles River Laboratories and LabCorp.
The U.S. is the biggest market for preclinical trial outsourcing as several biopharmaceutical companies prefer outsourcing their preclinical trials to CROs based in the U.S. to seek benefit from the Investigational New Drug (IND) application, approved by the FDA.
In addition, many life sciences and biopharmaceutical companies such as Pfizer Inc. and Eli Lilly and Company are present in the North American region. Presence of these companies in this region is also expected to impede the preclinical trial outsourcing market.
No credit card required*
Horizon in a snapshot
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more
Preclinical CRO Market Scope
Preclinical CRO Market Companies
| Name | Profile | # Employees | HQ | Website |
|---|
North America preclinical cro market size, by country, 2018-2030 (US$M)
North America Preclinical CRO Market Outlook Share, 2023 & 2030 (US$M)
Related industry reports
Related regional statistics
Sign up - it's easy, and free!
Sign up and get instant basic access to databook, upgrade
when ready, or enjoy our
free plan indefinitely.
Included in Horizon account
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more


