Copper Tubing - An Evolving Trend In Medical Gas Supply System
U.S. Medical Copper Tubing Market: Segmentation
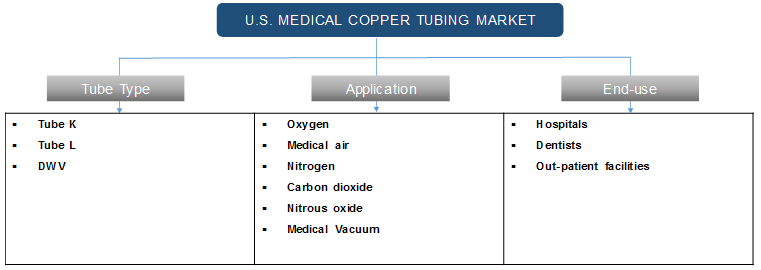
Medical copper tubing system is used for the supply of oxygen, medical air, nitrogen, carbon dioxide, nitrous oxide, medical vacuum, and other gases. Type K, Type L, and DWV are types of medical copper tubing and these are designed on the basis of ASTM standards. Each type indicates a series of sizes with varied wall thicknesses. Type K tubing has thicker walls than Type L tubing and the inner diameter depends on wall thickness and tube size. These tubing are widely used in various healthcare facilities including hospitals and outpatient healthcare facilities.
The medical copper tubing products should meet the regulatory standards including ASTM B819 and CSA. The ASTM B819 standard establishes the requirements for wall thickness range of specially cleaned & straight span of seamless copper tube suitable for gas supply systems. Also, these tubes should be installed in conformance with the National Fire Protection Association Standard 99, Gas and Vacuum Systems Standard 99C, Standard for Hypobaric Facilities Standard 99B, and CSA Standard Z 305.1/Z 7396.1.The compliance with these regulations helps gain trust of the customers by ensuring the safety and quality of the products, which boosts market growth.
The manufacturers have focused on strengthening their distribution channel by expanding their distribution and service centers. The most typical distribution channel for the medical copper tubing market is “manufacturer - supplier - distributer - customer.” The vendors prefer to market their products to customers directly through their own suppliers and distribution centers. The companies expand in specific territories through their service partners to provide better services to their customers. Thus, these inorganic strategies help the companies to uphold a large service force and build a strong product distribution pipeline.
There are many local and global vendors present in the U.S. medical copper tubing market. Some key players of this market include Mueller Industries, Inc.;Cambridge-Lee Industries LLC;BeaconMedaes;J & D Tube Benders, Inc.;Cerro Flow Products LLC;Samuel, Son & Co., Limited;C&H Medical (Guangzhou) Co., Ltd.;Wieland Copper Products LLC;KME Germany GmbH & Co KG;UACJ Corp.;Amico Group of Companies; and The Lawton Tube Co. Ltd.
Presence of advanced and sophisticated healthcare infrastructure with high per capita healthcare spending in the U.S. is expected to boost the market growth. However, innovation-led nature of the market assists sustainability of the new entrants that are subjected to the low entry and exit barriers. Some of the major factors attributing to the upsurge in the usage of copper tubing in medical application are increasing advancements in healthcare infrastructure, compliance with regulatory framework for manufacturing of devices, as well as the corrosion resistant and antibacterial properties of copper. However, risk associated with installation of copper tubing along with intense competition among the copper tubing vendors is expected to hinder market growth.
 In-depth report on U.S. medical copper tubing market by Grand View Research:
In-depth report on U.S. medical copper tubing market by Grand View Research:
https://www.grandviewresearch.com/industry-analysis/us-medical-copper-tubing-market