Congestive Heart Failure Treatment Devices Market: Emerging Technology In Healthcare
The Congestive Heart Failure (CHF) treatment devices market is highly competitive with the top market players accounting for almost similar revenue shares. Companies have adopted strategies including mergers & acquisitions and strategic alliances to gain a competitive advantage. Additionally, market players are more focused on the development of technologically advanced products, which deliver greater accuracy and efficiency. Entry of new players is posing a threat to the existing market players thereby maintaining the industry rivalry on the higher side.
Medtronic plc dominated the global CGF treatment devices market with a wide portfolio and global reach. The company has been witnessed to expand its portfolio as well as regional presence through mergers and acquisitions. Boston Scientific Corporation is also involved in expanding their global presence. New entrants are expected to maintain a competitive edge in terms of pricing strategies and operation costs in order to sustain the competition and ensure sustainability.
Driving forces of the market include:
-
Rising prevalence of cardiovascular diseases (CVD)
-
Growing geriatric population base
-
Favorable reimbursement policies
-
Advent of technological advanced products
-
New product approvals
The incidence rate of CVDs in the developing regions, such as the Asia Pacific and Latin America, is on the rise. As per the WHO, out of 17.5 million, more than 75.0% deaths are reported in the low and medium income countries. This forms the key target region for CHF treatment devices. The economic developments, favorable government reforms, changing foreign policies, and increasing focus on quality healthcare are anticipated to support the market growth.
Favorable reimbursement policies lead to higher adoption rates amongst patients in the U.S., Canada, and UK, enabling further market penetration of these devices. This trend is also witnessed in the developing regions of Asia Pacific. For instance, Japan’s National Health Insurance system provides reimbursement for all medical devices.
Advent of remote patient monitoring has enabled faster clinical decision-making for patients suffering from arrhythmias, thereby saving many lives. Furthermore, the development of subcutaneous ICDs escape the risk associated with using intravascular leads.
Companies today are more focused on developing high-utility products, which can deliver personalized care. These advancements are improving accuracy, providing additional capabilities to enhance the workflow, and facilitating error reduction.
Approval of existing products in new geographies is expected to boost market potential over the forecast period. For instance, Jarvik Heart Inc., received pre-market approval (Shonin) from a Japanese Pharmaceutical and Medical Devices Agency for the Jarvik 2000 ventricular assist device. Through this approval Jarvik aims to enter the Asian market.
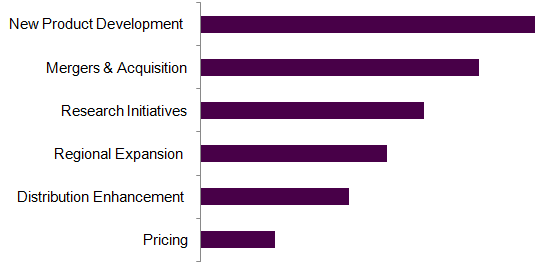
The chart below provides the current strategic framework for the congestive heart failure treatment devices market based on the industry players.
 In-depth report on global congestive heart failure treatment devices market by Grand View Research:
In-depth report on global congestive heart failure treatment devices market by Grand View Research: