- Home
- »
- Market Trend Reports
- »
-
Competitive Analysis Of Ophthalmic Devices In The U.S.
Report Overview
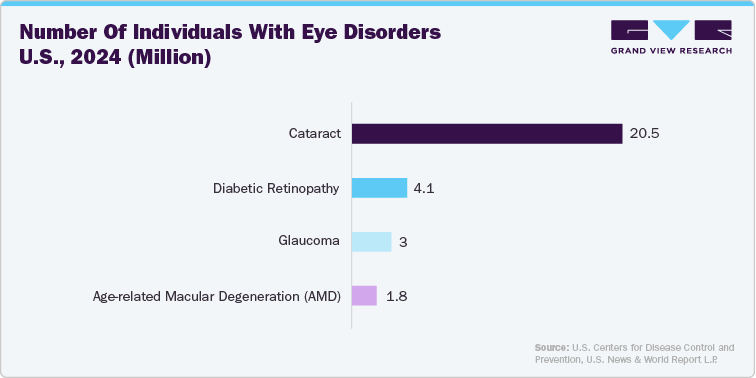
The competitive analysis of the ophthalmic devices industry in the U.S. is driven by several key factors. The aging population significantly impacts market growth as conditions like cataracts, glaucoma, and age-related macular degeneration become more prevalent, increasing the demand for diagnostic and surgical solutions. Technological advancements are crucial drivers, with companies continually innovating to offer more precise, efficient, and minimally invasive devices. For instance, advancements in femtosecond lasers and intraocular lenses (IOLs) have revolutionized cataract surgery, while minimally invasive glaucoma surgery (MIGS) devices are transforming glaucoma treatment.
The FDA recently qualified the American Academy of Ophthalmology's Assessment of Intraocular Lens Implant Symptoms (AIOLIS) as a Medical Device Development Tool (MDDT) in May 2024. This designation enables AIOLIS to evaluate patients' visual disturbances after premium IOL cataract surgery, filling a critical gap in assessing patient symptoms compared to traditional IOLs. This is the second MDDT approved under the Academy's guidance, following the Patient-Reported Outcomes with LASIK Symptoms and Satisfaction (PROWL-SS). Researchers now have a tool for regulatory decision-making that emphasizes the patient's perspective on premium IOLs.
The AIOLIS assessment tool was developed through a multi-year collaboration involving the American Academy of Ophthalmology, UCLA, Dr. Ron D. Hays, RAND, and four IOL manufacturers: Bausch & Lomb, Alcon, Johnson & Johnson, and Carl Zeiss Meditec AG. It was field-tested by 20 cataract surgeons at various sites in the U.S. and two international locations. The expressions that support our sayings are:
“This uniquely collaborative journey allowed us to recognize the great value in understanding the visual outcomes of premium IOL cataract surgery from patients' observations and to produce an instrument that reflects those findings. At the end of the day, the devices that the manufacturers provide and what clinicians do at surgery must primarily benefit the patient and satisfy their visual needs.”
-Samuel Masket, MD, lead for the AIOLIS development and clinical professor of ophthalmology at the David Geffen School of Medicine at UCLA
Competitive landscape of ophthalmic devices in the U.S. market, focusing on key players and their areas of expertise:
Company Name
Key Areas of Expertise
Alcon
Cataract and refractive surgery, contact lenses, and vision care products.
Johnson & Johnson Services, Inc.
Contact lenses and intraocular lenses (IOLs).
Bausch Health Companies Inc.
Ophthalmic pharmaceuticals, contact lenses, and surgical devices.
TOPCON CORPORATION
Diagnostic equipment and software for ophthalmology.
Glaukos Corporation
Minimally invasive glaucoma surgery (MIGS) devices.
Hoya Corporation
Intraocular lenses (IOLs) and optical lenses.
Lumenis Ltd.
Laser systems for ophthalmic and other medical applications.
Ziemer Ophthalmic Systems
Lasers for refractive and cataract surgery.
Nidek Co. Ltd.
Diagnostic devices and surgical equipment for ophthalmology.
CooperVision Inc
Contact lenses and related products.
Haag-Streit Holding AG
Diagnostic instruments and surgical microscopes for ophthalmology.
STAAR Surgical
Implantable Collamer Lenses (ICLs) for vision correction.
SWOT analysis of major market participants
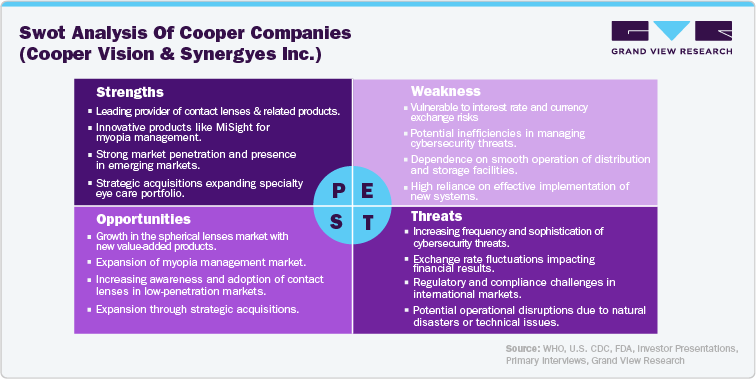
SWOT analysis is crucial for understanding market dynamics and developing strategies for growth, particularly in competitive sectors like the U.S. ophthalmic devices. Here is a SWOT analysis for Cooper Companies Inc., which highlights their competitive advantages, areas for improvement, market expansion possibilities, and external challenges, providing strategic insights for growth.
Sustainability Initiatives
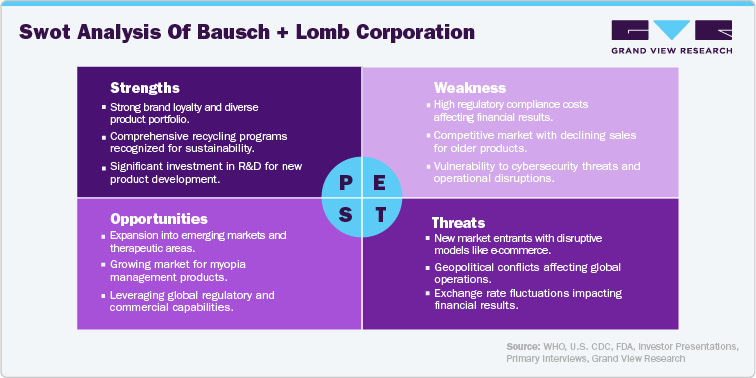
Ophthalmic device manufacturers are increasingly recognizing the importance of sustainability and are implementing various policies and commitments. These efforts encompass reducing environmental impact, promoting ethical sourcing, and extending product lifecycle. Many companies focus on minimizing waste through efficient production processes, using recyclable materials, and developing end-of-life management programs. In addition, there's a growing emphasis on energy conservation and carbon emission reduction throughout the supply chain. Some manufacturers are also investing in research and development to create more sustainable and biodegradable ophthalmic devices. Here is a comparison table between Hoya Corporation and Alcon, focusing on their sustainability policies and commitments:
Feature
Hoya Corporation
Alcon
Innovation
Aim to resolve global social issues through innovation in the business.
Focus on social impact and sustainability (SIS) as essential aspects of long-term business performance.
Corporate Governance
Commit to highly transparent and fair corporate management by building trust-based relationships with key stakeholders through consistent dialogue.
Consider investors' and other market participants' judgments based on social impact and sustainability performance.
Environmental Responsibility
Aim to minimize environmental externalities in the business activities to ensure a healthy global environment for future generations.
Track and report corporate responsibility program, including efforts on climate change and sustainability.
Human Rights
Respect the human rights of all individuals involved in business activities and strive to prevent any abuse within the supply chain.
Focus on human rights, diversity, and inclusion as key elements of sustainability assessments.
Work Environment
Create a work environment that promotes diversity and inclusion, with an emphasis on employee wellbeing and high morale and motivation to create new value.
Emphasize diversity, inclusion, ethics, and compliance with laws, ensuring the public's ability to access products.
Stakeholder Engagement
Build trust-based relationships with key stakeholders through consistent dialogue.
Respond to investors' demands for ambitious SIS goals and robust disclosures on progress toward these goals.
Goals and Reporting
Focus on creating new value by maintaining high morale and motivation among employees.
Establish and announce goals related to SIS matters, adapting tracking and reporting to various SIS frameworks.
Recycling initiatives adopted by ophthalmic device manufacturers in the U.S.
With a growing consciousness toward sustainability, many companies in the U.S. have initiated recycling programs for ophthalmic devices, not only addressing environmental concerns but also contributing to the expansion of the market by promoting responsible consumption and resource utilization. For instance:
-
Alcon collaborates with Plastic Bank to counteract the plastic used in its surgical and vision care products. For each ton of plastic in their UltraSert, AutonoMe systems, and contact lenses, an equivalent amount of ocean-bound plastic is collected. In 2023, this partnership removed around 67 million plastic bottles
-
Bausch + Lomb’s ONE by ONE and Biotrue Eye Care Recycling programs were awarded “Sustainability Service of the Year” in 2023, having collected over 76 million used items since 2016
-
CooperVision’s recycling program, started in August 2019, focuses on reducing plastic waste. These efforts support environmental protection and bolster brand reputation and customer loyalty by showcasing a commitment to sustainability
Strategic Initiatives
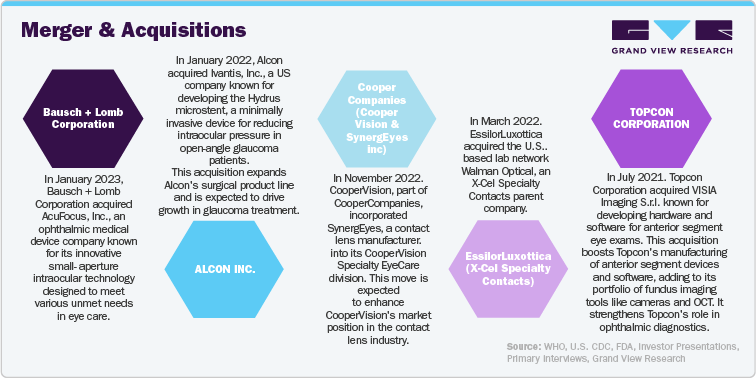
Major ophthalmic device companies employ organic and inorganic strategies to address customer needs and drive growth in the U.S. These strategies include developing new products, forming strategic collaborations, and pursuing acquisitions and mergers. By focusing on innovation and expanding their product offerings through these approaches, companies aim to meet the evolving demands of their customers and enhance their market presence. Such efforts are crucial for advancing the field of ophthalmic devices and ensuring that emerging needs in eye care are effectively addressed, ultimately supporting the expansion and competitiveness of the ophthalmic devices sector in the U.S.
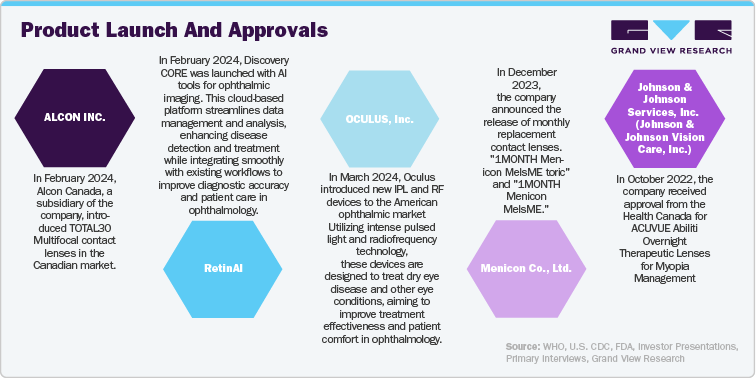
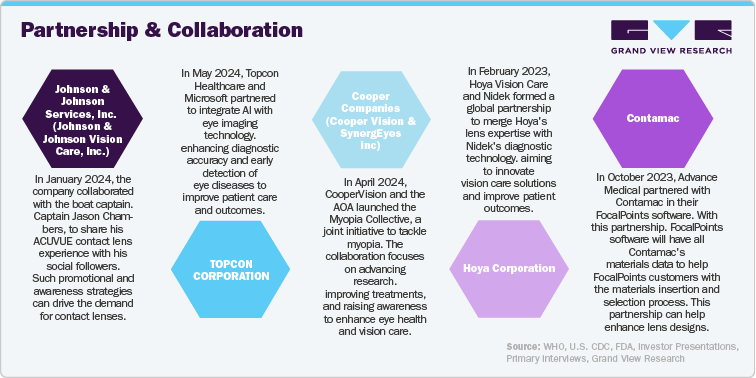
Perspectives from industry leaders across different companies like STAAR Surgical, CooperVision, NIDEK CO., LTD., Glaukos Corporation are as follows:
“Our goal as ophthalmologists should not just be to treat eye diseases, but to help our patients embrace all life has to offer. By doing our part to adopt eco-friendly business practices, such as reducing our carbon footprint through ICL implantable lenses, we help our patients maintain an active lifestyle with healthy eyes, enabling them to enjoy the world around them.”
-Dr. Ignacio López-Marín Espigares Ophthalmologist - Granada, Spain
“Disrupting the status quo and establishing a new standard of care for children with myopia is a monumental task, one that requires collective effort. The AOA’s full and active participation in The Myopia Collective alongside CooperVision is an unmistakable sign of how critical this issue is to the eye health and vision care for Americans both today and in the decades to come,”
- Michele Andrews, OD, Vice President, Professional and Government Affairs, Americas, CooperVision.
“The NT-1 series offers optimal products that satisfy a wide range of customer needs to meet clinical care. Leveraging our expertise in optical technology and engineering, we will continue to develop products that address our customers’ needs for efficient patient throughput while maintaining a small device footprint ensuring good patient flow at clinical facilities and optical shops.”
- Motoki Ozawa, President and CEO of NIDEK CO., LTD.
“The FDA approval of iDose TR represents a significant milestone for Glaukos following an extensive pioneering journey since the inception of the original idea nearly 15 years ago. Today’s approval ushers in a new era of interventional glaucoma therapy by enabling a more proactive and reliable approach for patients in need, We believe iDose TR can be a transformative, novel technology able to fundamentally improve the treatment paradigm for patients with open-angle glaucoma or ocular hypertension. We are grateful to the clinical investigators and study participants in the clinical trials for their instrumental roles in helping us reach this important advancement for glaucoma patient care. At Glaukos, we are relentlessly focused on delivering novel therapies for chronic eye diseases and now iDoseTR has the potential to redefine the standard of care for patients in the U.S. affected by open-angle glaucoma and ocular hypertension.”
- Thomas Burns, Glaukos chairman and chief executive officer.
Share this report with your colleague or friend.
GET A FREE SAMPLE
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities.
Contact us now to get our best pricing.
![esomar icon]()
ESOMAR certified & member
![ISO]()
ISO Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
-
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


