- Home
- »
- Market Trend Reports
- »
-
Tuberculosis Diagnostics: Trends And Competitive Analysis
Tuberculosis (TB) continues to pose a major global health challenge, further complicated by the emergence of extensively drug-resistant (XDR) and multidrug-resistant (MDR) strains. Each year, 10 million people worldwide contract TB, an infectious disease that primarily affects the lungs but can also target other parts of the body. Annually, TB claims 1.5 million lives, making it the leading infectious killer. It is the primary cause of death among people living with HIV and significantly contributes to global antimicrobial resistance.
The trends and competitive analysis report, compiled by Grand View Research, is a collection of the trends and competitive scenario in more than 20 countries. Qualitative information regarding the trends, government initiatives, funding landscape, competitive strategies, existing competition, and pipeline analysis will be provided in the report. Within the purview of the database, such information is systematically analyzed and provided in the form of outlook reports and summary presentations on individual areas of research.
Tuberculosis Diagnostics: Trends And Competitive Analysis Report Scope
Attributes
Details
Areas of Research
Industry trends, market opportunity, ease of doing business across countries, competitive analysis
Report Representation
Consolidated report in PDF format
Country Coverage
20+ Countries
Highlights of Report (Competitive Landscape, by country)
- End TB Strategy
- Funding Programs
- R&D Funding
- Company Strategy Mapping
- Existing Diagnostic Techniques SWOT
- Recent Developments
- Pipeline Landscape
- Companies Planning to Launch TB Diagnostic Products
Tuberculosis Diagnostics: Trends And Competitive Analysis Coverage Snapshot
Recent trends indicate progress in diagnostics, treatment, and prevention, fueled by technological advancements, regulatory changes, and increased funding. Recognizing the need for innovative approaches to prevent, diagnose, and treat TB to meet the 2030 elimination goal, UN member states made new commitments at the 2023 UN High-Level Meeting on TB. They agreed to raise the annual funding target for TB research to $5 billion by 2027, more than doubling the $2 billion target set in 2018.
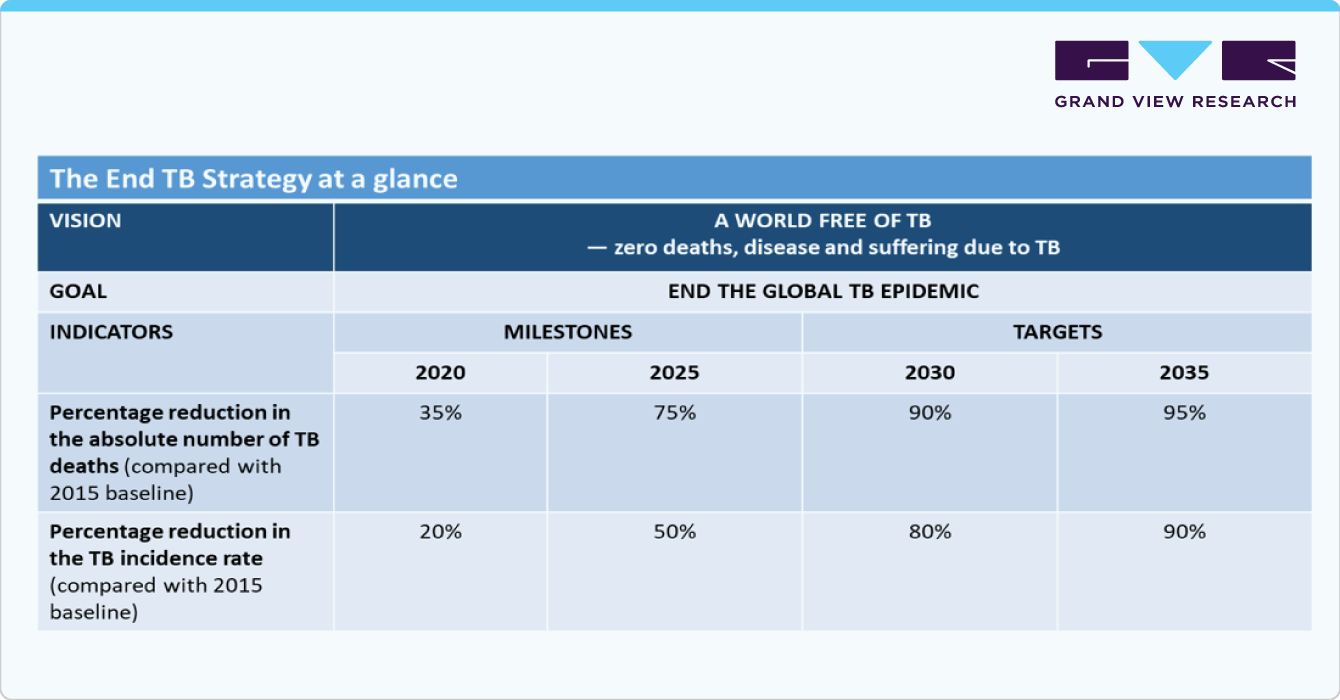
The WHO's End TB Strategy underscores the importance of integrating innovative diagnostic tools to improve early and accurate TB detection. Molecular diagnostics, such as the GeneXpert MTB/RIF assay and next-generation sequencing, are crucial for rapidly diagnosing TB and detecting drug resistance. Additionally, in April 2022, the WHO evaluated Tuberculosis antigen-based skin tests (TBST), a new class of tests for diagnosing TB infection. These tests have been found to be feasible, accurate, cost-effective, and acceptable, offering an alternative to the tuberculin skin test (TST) and Interferon-Gamma Release Assays (IGRAs).
FUNDING PROGRAMS TO PROMOTE TB DIAGNOSIS
-
The U.S. government has significantly increased its funding for TB initiatives, from USD 233 million in 2013 to USD 406 million in 2023. Investments in TB screening and preventive treatment have shown significant health & economic benefits, with a return on investment of up to USD 39 for every dollar spent. Hence, expanding investments in TB diagnosis programs is essential to improve access to diagnosis, treatment, and prevention to eliminate the TB epidemic by 2030.
-
The Global Fund plays a significant role in the fight against TB, contributing 76% of total international funding for TB. By June 2023, it had allocated USD 9.2 billion toward TB prevention and treatment programs. Additionally, an extra USD 1.5 billion was allocated for TB/HIV initiatives, further demonstrating the Global Fund's commitment to addressing the TB epidemic.
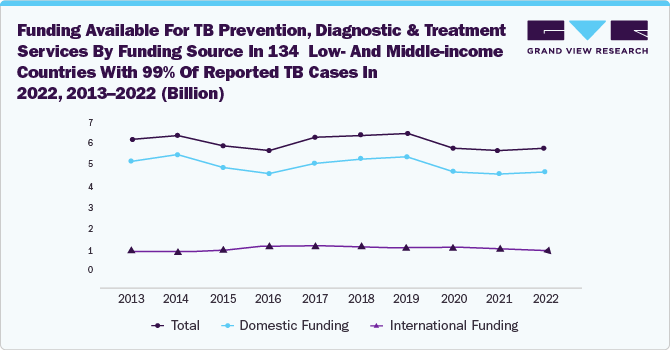
In addition to awareness programs and government initiatives, strategic activities by key market players and the launch of technologically advanced products will create lucrative opportunities in the TB diagnostics market.
Companies
Year
Month
Details
Oxford Immunotec
2024
March
Revvity, Inc. has announced the launch of the Auto-Pure 2400 liquid handler from Allsheng for use with the T-SPOT.TB test. The Auto-Pure 2400 platform is user-friendly and designed to streamline lab workflows. By combining the accuracy of the T-SPOT.TB test with the efficiency of the Auto-Pure 2400 system, this powerful solution offers significant benefits to labs, clinicians, and patients alike.
Oxford Immunotec
2023
November
Revvity launched its T-SPOT TB test for latent TB screening in India, during the 46th Edition of MICROCON in Lucknow. The company emphasizes that it is the sole commercially available IGRA based on ELISPOT technology to have received FDA approval.
QIAGEN
2023
February
QIAGEN N.V. announced the certification of its QFT-Plus test under the European Union’s 2017/746 IVD Medical Devices Regulation (IVDR), replacing the 98/79/EC In Vitro Diagnostic Directive (IVDD). QFT-Plus is a leading TB blood test endorsed by the WHO, aiding in the indirect detection of TB-causing bacteria. This certification follows the IVDR CE-marking of QIAGEN’s ipsogen JAK2 RGQ PCR Kit, NeuMoDx Systems, and reagents in late 2022.
Currently used diagnostic techniques, recognized by the WHO, with the associated strengths and weaknesses
Diagnostic method
Sensitivity (95% CI)
Specificity (95% CI)
Strengths
Weaknesses
Bacterial culture
100%
100%
+ Gold standard of sensitivity and specificity
+ Drug sensitivity testing can take place in tandem (through use of drug diffusion disks)
+ Cheaper than molecular/immunological methods- Requires high level biosafety containment laboratory
- Generation of results is time-consuming (>28 days)MGIT (Becton Dickinson and Company, U.S.A.)
86%-93%
99.99%
+ Faster than conventional culture
+ High degree of specificity and sensitivity- More expensive than conventional culture
- Requires specialist training
- Method is labour intensive
- Requires high-containment labRecent developments of Novel Technologies
-
ddPCR
-
Recent advancements have incorporated emerging technologies such as digital droplet PCR (ddPCR), which partitions the amplification reaction seen in PCR, providing absolute quantification of gene expression with higher sensitivity than qPCR. Capable of detecting single DNA copies, ddPCR has been used to monitor cell populations for drug resistance and can test for Mtb infection in sputum and blood samples, making it valuable for pulmonary, extrapulmonary, LTBI, and active TB. However, its primary drawback is its prohibitive cost.
-
-
CRISPR
-
Next-generation sequencing techniques
-
MicroRNA detection
-
eNose
-
Raman spectroscopy
- AI processing
Share this report with your colleague or friend.
GET A FREE SAMPLE
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities.
Contact us now to get our best pricing.
![esomar icon]()
ESOMAR certified & member
![ISO]()
ISO Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


