- Home
- »
- Market Trend Reports
- »
-
Rise Of Single-Use Devices In General Surgery Industry
Executive Summary
The use of disposable and single-use devices in general surgery has risen dramatically, driven by concerns about patient safety and the convenience of pre-sterilized instruments. However, this trend is facing challenges due to high costs and environmental impact. As a result, a counter-trend is emerging: the reprocessing of certain single-use devices for safe reuse. This report explores these trends and their potential impact on the future of general surgery.
Factors Driving The Rise Of Single-Use Devices
Patient Safety: The primary driver has been the emphasis on reducing the risk of Hospital-Acquired Infections (HAIs). Single-use devices eliminate the possibility of cross-contamination that can occur during reprocessing of reusable instruments.
Convenience: Single-use devices are often pre-sterilized and readily available, saving time and resources in the operating room. This can be especially beneficial in minimally invasive surgeries.
Technological Advancements: New technologies often come packaged as single-use devices, offering improved functionality and efficiency compared to reusable alternatives. Manufacturers can focus on creating specialized, disposable devices for specific procedures, leading to more precise and effective surgeries.
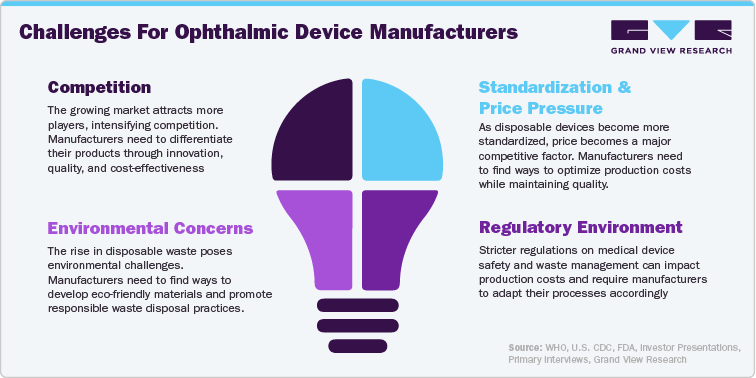
Challenges Of Single-Use Devices
Cost: The widespread use of single-use devices can significantly increase healthcare costs for hospitals and patients.
Environmental Impact: The massive amount of medical waste generated by disposable devices creates a significant environmental burden.
Hospitals began reusing single-use medical devices to save cost. This practice is controversial because of safety concerns. The FDA stepped in to regulate this practice. They require hospitals and reprocessing companies to meet the same safety standards as original manufacturers. This means following strict procedures to clean and sterilize devices and proving they work just as well as new ones. Hospitals have options: stop reusing, follow the FDA rules, or outsource reprocessing. The FDA's guidance doesn't apply to all devices or settings, and regulations are constantly evolving.
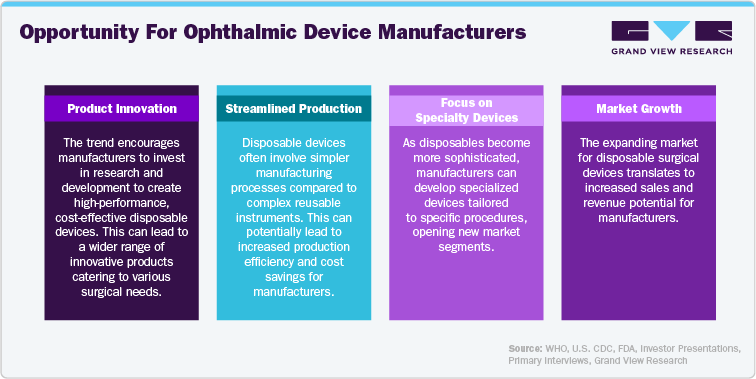
Rise Of Reprocessing
Cost Savings: Due to the cost concerns, there's a growing interest in reprocessing certain single-use devices.
Safety Concerns: Improper reprocessing can compromise the sterility and functionality of the device, potentially jeopardizing patient safety.
Regulations: Strict guidelines exist for reprocessing to ensure the devices meet safety standards.
Market Growth: The market for reprocessing single-use medical devices is projected to grow due to its cost-saving potential.
Regulatory Framework
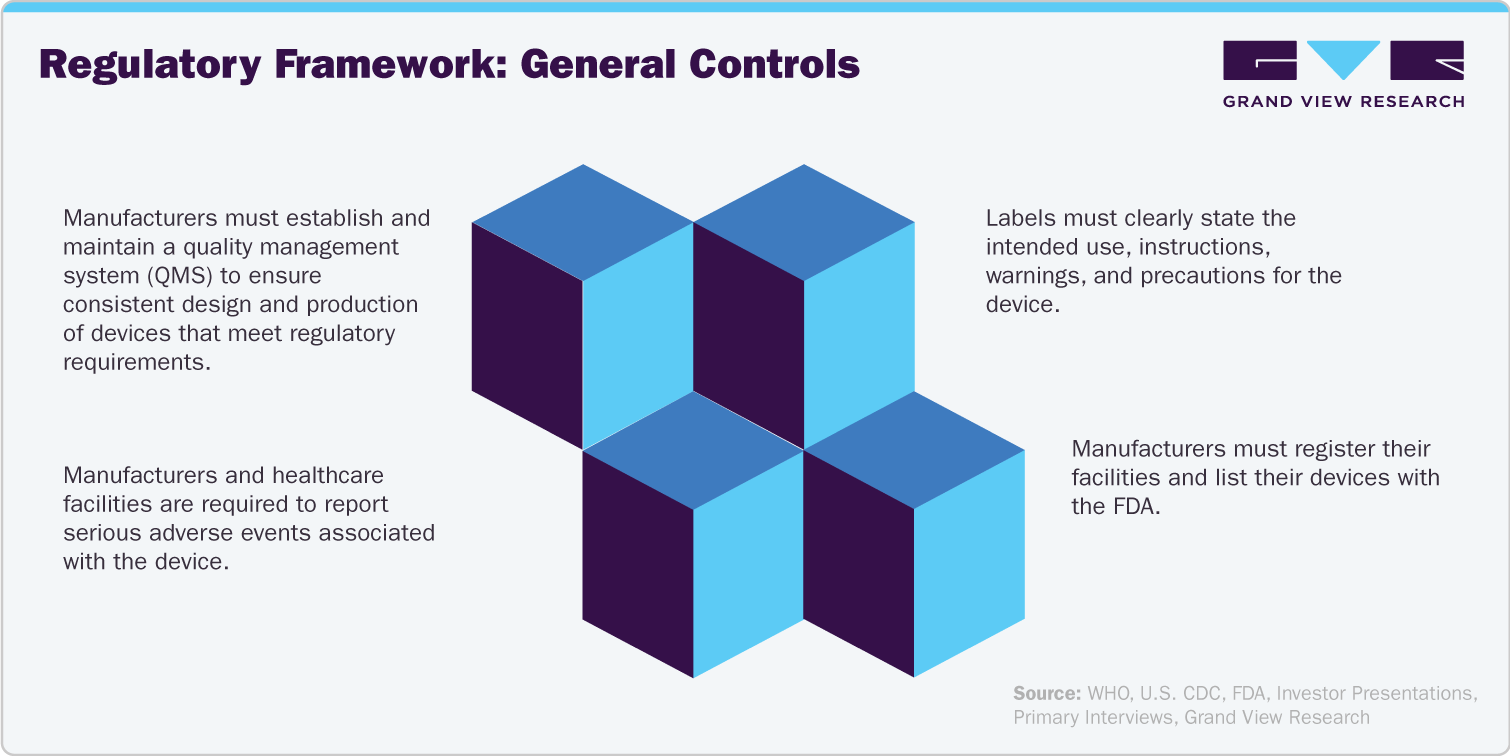
The regulatory framework for single-use general surgery devices is primarily overseen by the Food and Drug Administration (FDA) in the U.S. Here's a breakdown of the key aspects:
Premarket Requirements:
Classification: The FDA classifies medical devices based on their risk. General surgery devices can fall into Class I (lowest risk), Class II (moderate risk), or Class III (highest risk).
Clearance or Approval: Depending on the classification, manufacturers need to obtain premarket clearance (510(k)) or premarket approval (PMA) from the FDA. This demonstrates the device is safe and effective for its intended use.
Specific Considerations For Single-Use Devices
Reprocessing: The FDA generally discourages the reuse of single-use devices. If a hospital or third-party company wants to reprocess a single-use device, they are considered "manufacturers" and must comply with all the regulations mentioned above for original manufacturers.
Future Outlook
Prioritizing Patient Safety: Safety will remain paramount, with single-use devices being the preferred option when reprocessing poses a risk.
Evaluating Reprocessing Options: Hospitals may consider reprocessing specific devices where validated protocols exist and cost savings outweigh concerns.
Focus on Sustainability: There's a growing focus on developing more environmentally friendly disposable devices or finding ways to safely reprocess a wider range of devices.
The use of single-use devices in general surgery is likely to continue, but with increasing emphasis on responsible reprocessing and sustainable solutions. The future may see a hybrid approach, with single-use devices remaining dominant for high-risk procedures and reprocessing becoming more common for select devices where safety and cost-effectiveness can be balanced.
Share this report with your colleague or friend.
GET A FREE SAMPLE
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities.
Contact us now to get our best pricing.
![esomar icon]()
ESOMAR certified & member
![ISO]()
ISO Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


