- Home
- »
- Market Trend Reports
- »
-
Platform Trials Market Trend Analysis
Report Overview
The platform trials market has emerged as a pivotal innovation in clinical research, transforming novel drug development processes for several pharmaceutical companies, biotechnology firms, and Contract Research Organizations (CROs). Unlike traditional trials, platform trials evaluate multiple therapies within a single comprehensive trial infrastructure, offering numerous advantages such as efficiency, speed, and cost-effectiveness. Platform trials provide a streamlined pathway to bring therapies to market faster while minimizing risk through adaptive designs for several industry players, including drug developers, research institutions, and healthcare investors. This study will provide the growth potential, market drivers, and challenges in organizing and managing profitable platform trials for industry stakeholders.
Efficiency in Drug Development is a major driver in the adoption of platform trials. These trials allow multiple therapies to be assessed concurrently under one trial infrastructure, reducing costs and speeding up the time-to-market for new treatments. By enabling adaptive designs that let sponsors drop ineffective drugs early and prioritize promising ones, platform trials help biopharmaceutical companies and CROs streamline clinical research. This efficiency lowers the financial risks and operational complexities associated with traditional trials, making platform trials a valuable tool for companies aiming to optimize their R&D investments.
Additionally, growing focus on precision medicine is creating an increasing demand for platform trials. As therapies become more targeted, particularly in oncology and rare diseases, the need to evaluate drugs on specific patient subpopulations has intensified. Platform trials offer a flexible framework to simultaneously evaluate multiple therapies or combinations across varied genetic and clinical profiles. This approach allows research institutes and biopharmaceutical companies to gather valuable data on how different treatments perform in personalized settings, accelerating the development of more effective, patient-specific therapies while maximizing resource utilization.
Platform Trials Vs Traditional Clinical Trials Workflow Analysis
In the evolving landscape of clinical research, platform trials are emerging as a preferred alternative to traditional clinical trials, offering increased efficiency and adaptability. The core distinction between these two models lies in trial design, workflow management, and flexibility, making platform trials highly attractive for biopharmaceutical companies, Contract Research Organizations (CROs), and research institutes.
Traditional Clinical Trials typically follow a linear workflow where a single treatment is evaluated for a specific indication. Each novel therapy requires a separate trial, leading to inefficiencies in patient recruitment, excessive costs, and extended timelines. Once the trial is complete, it is closed, and the infrastructure must be re-established for subsequent studies.
In contrast, platform trials utilized a master protocol to test multiple therapies simultaneously for the same disease. This adaptive design allows the addition or removal of therapies based on provisional data, significantly reducing time-to-market and costs. Platform trials create an ongoing infrastructure by centralizing recruitment, trial sites, and data management that can be reused across different treatment arms to minimize dismissals. Moreover, platform trials accelerate precision medicine, allowing therapies to be evaluated on targeted subgroups, aligning with modern personalized treatment strategies. This approach enhances trial efficiency and improves patient outcomes.
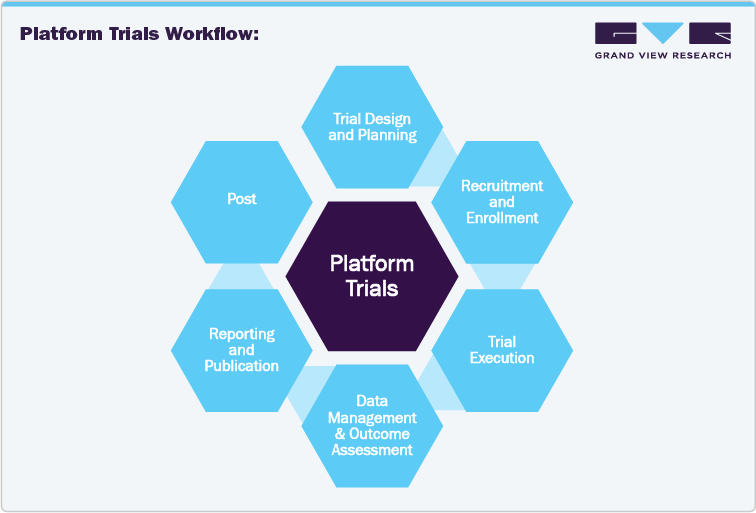
For biopharmaceutical companies, platform trials are more cost-effective and risk-mitigating. Ineffective drugs can be dropped early, and promising candidates can be advanced without restarting the entire trial process. This flexibility ensures optimal resource allocation and faster identification of successful treatments. CROs and outsourcing firms gain an advantage from the continuous demand for trial management services and long-term contracts, as platform trials are dynamic and last over several years. Thus, as platform trials gain traction, companies looking to stay competitive in drug development should consider adopting this innovative trial design.
Therapeutic Area Insights
Growing demand for innovative and effective cancer treatments has significantly increased the utilization of platform trials. These trials offer a streamlined approach to testing multiple therapies across various cancer subtypes, optimizing resource allocation and expediting the development of targeted therapies. The ability to adapt trial designs based on real-time data allows for more efficient identification of promising treatments, addressing the complexity of cancer with precision. Moreover, growing platform trials for cancer treatments such as I-SPY 2 trial, an adaptive platform trial specifically designed to test multiple drugs simultaneously in patients with high-risk breast cancer will offer lucrative growth opportunities in the clinical trial landscape.
The COVID-19 pandemic has highlighted the critical need for active and responsive research models, drawing attention to platform trials as essential tools for pandemic preparedness and infectious disease research. Platform trials, such as ENSEMBLE, RECOVERY, and ACTIV, are designed to simultaneously test several potential treatments in hospitalized COVID-19 patients. Further, the growing adoption of these models among biopharmaceutical companies, CROs, and research institutes to enhance operational capabilities to respond to future pandemics, offers shared infrastructure and adaptive designs to accelerate the development of effective therapies and vaccines while managing risks more efficiently.
Regional Insights
North America region is witnessing rapid growth in the platform trials market. The regional growth is primarily attributed to ongoing advancements in AI, machine learning, and big data that streamline trial management and improve efficiency. Supportive regulatory frameworks, such as the U.S. FDA’s acceptance of adaptive trial designs, have accelerated adoption, reducing time to market for biopharmaceutical companies. Further, the availability of collaborative infrastructure through research institutes and public-private partnerships has further expanded trial capabilities, reduced costs, and shared resources. Additionally, the shift toward decentralized trials post-pandemic, along with increased demand for skilled workforce expertise in trial management, is anticipated to boost demand for the platform trial model in the region.
Case Study Insights:
Case Study 1.-RECOVERY Trial
-
Title: Randomised Evaluation of COVID-19 Therapy (RECOVERY)
-
Initiated: March 2020
-
Location: United Kingdom
-
Sponsor: University of Oxford
-
Objective: Evaluate various treatments for COVID-19 and determine their efficacy and safety.
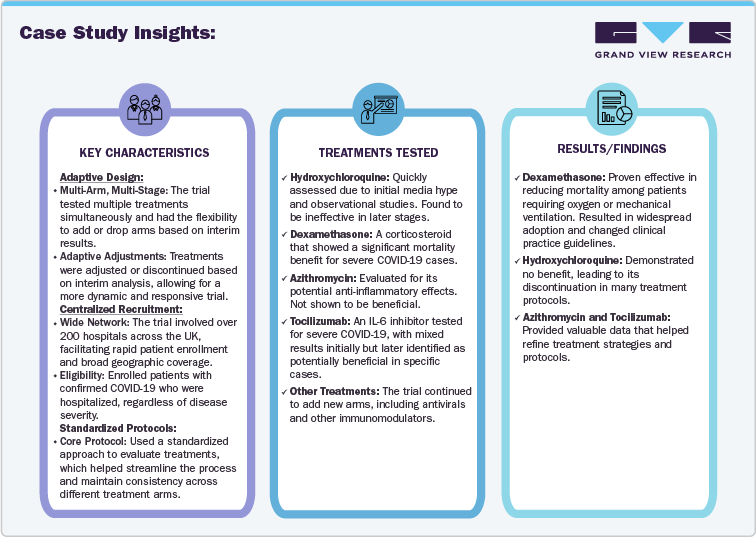
Impact on the Market:
-
Guidelines: RECOVERY’s findings influenced global treatment guidelines and informed clinical decision-making.
-
Speed: The trial’s adaptive design and centralized approach allowed for rapid testing of multiple treatments during a pandemic.
-
Platform Model: Established the effectiveness of platform trials in addressing urgent public health challenges, proving that such models can accelerate the evaluation of treatments and streamline the research process.
Furthermore, other aspects that shall be analyzed include the industry ecosystem, technology landscape, market dynamics, market entry strategies, revenue model analysis, trend analysis based on therapeutic areas, study design, and regional-level insights.
Share this report with your colleague or friend.
GET A FREE SAMPLE
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities.
Contact us now to get our best pricing.
![esomar icon]()
ESOMAR certified & member
![ISO]()
ISO Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
-
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


