Report Overview
The study of the North America Pharmaceutical Logistics Market Qualitative Assessment by State/Province, Good Manufacturing Practices (GMP), Good Distribution Practices (GDP), Regulatory Landscape & Compliances, CAPEX and OPEX Cost Analysis, and Investment Analysis, compiled by Grand View Research, offers a detailed qualitative assessment of the pharmaceutical logistics market across the U.S. and Canada. The study covers insights into the top 5 gross freezer spaces and usable freezer spaces in the U.S. The qualitative analysis of the key factors attributing to good manufacturing practices (GMP) in the pharmaceutical industry provides a gist of the GMP regulations across the U.S. and their impact on the manufacturers. Similarly, the qualitative analysis of the key factors attributing to the good distribution practices (GDP) in the pharmaceutical logistics market for leading market players provides a gist on the GDP compliances, certifications, and implementation by the pharmaceutical manufacturers across the U.S. The study also covers the qualitative analysis of GMP and GDP standards in the pharmaceutical cold storage market.
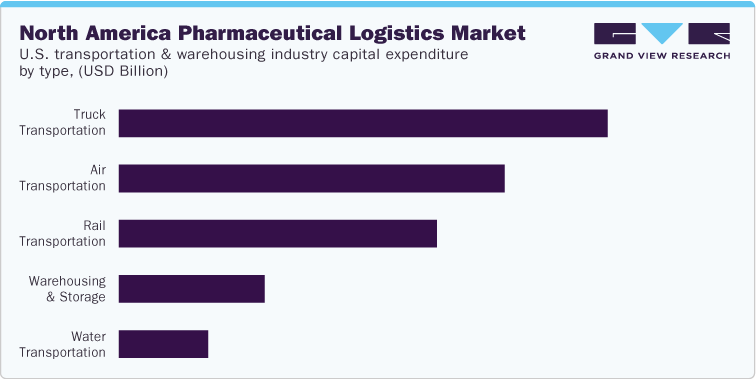
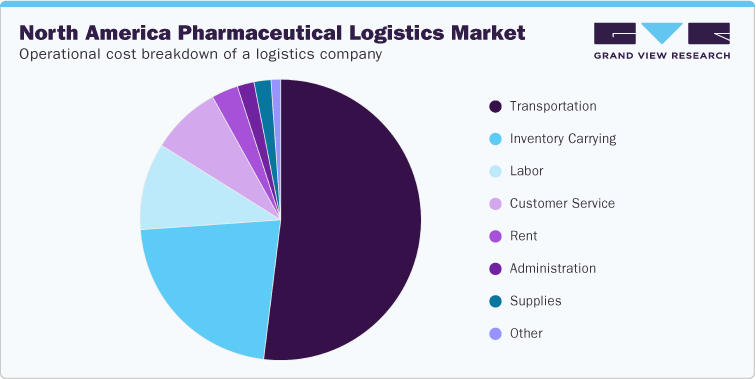
Further, the regulatory landscape & compliances section covers the qualitative analysis in terms of FDA/DEA inspection. In terms of the CAPEX and OPEX analysis, the study covers insights on the breakdown costs of pharmaceutical logistics by functions, including transportation costs, inventory costs, warehousing costs, and administrative costs; and modes, including rail, air, maritime, and trucking. Moreover, the study also covers insights into the capital expenditure in the transportation and warehousing industry in the U.S. and the breakdown of the operational costs of a logistics company.
North America Pharmaceutical Logistics Market Coverage Scope
|
Report Offering
|
Details
|
|
Qualitative Assessment of North America Pharmaceutical Logistics Market, By State/Province
|
It includes the qualitative analysis of the pharmaceutical logistics market across the following regions
- U.S.
- Northeast U.S.
- Southeast U.S.
- West U.S.
- Midwest U.S.
- Southwest U.S.
- Canada
- Atlantic provinces
- Central Canada
- Prairie
- West coast
- Northern Canada
|
|
Good Manufacturing Practices (GMP) and Good Distribution Practices (GDP) in the Pharmaceutical Logistics Market
|
- Qualitative analysis of the GMP and GDP in the pharmaceutical logistics market
- Qualitative analysis of GMP and GDP standards in the pharmaceutical cold storage market
|
|
Regulatory Landscape & Compliances
|
It includes the qualitative analysis of the regulatory landscape & compliances in terms of FDA/DEA inspection
|
|
CAPEX and OPEX Analysis
|
It includes the CAPEX and OPEX cost analysis and investment analysis in the pharmaceutical logistics market
|
North America Pharmaceutical Logistics Market Qualitative Assessment
|
Report Attribute
|
Details
|
|
Area of Research
|
- North America Pharmaceutical Logistics Market Qualitative Assessment By State/Province, Good Manufacturing Practices (GMP), Good Distribution Practices (GDP), Regulatory Landscape & Compliances, CAPEX and OPEX Cost Analysis, and Investment Analysis
|
|
Report Representation
|
- Consolidated report in PDF format
|





