- Home
- »
- Market Trend Reports
- »
-
Facial Injectables: Top Brands And Competitive Landscape
Report Overview
Facial injectables continue to grow across regions and countries. The growing access to services through aesthetic clinic chains, medical spas, and beauty bars, alongside the accelerating consumer purchasing power, has allowed facial injectables to penetrate emerging markets. Furthermore, changing consumer attitudes toward aesthetics have increased awareness and acceptance of these products, allowing them to penetrate newer market segments, such as men and millennials.
The brand and competitive analysis report, compiled by Grand View Research, is a collection of the trends and competitive scenarios in more than 20 countries. Qualitative information regarding the trends, competitive strategies, existing competition, and pipeline analysis will be provided in the report. Within the purview of the database, such information is systematically analyzed and provided in the form of outlook reports and summary presentations on individual areas of research.
Facial Injectables: Trends and Competitive Analysis Report Scope
Attributes
Details
Areas of Research
Industry trends, market opportunity, aesthetic penetration across countries, competitive and product revenue analysis
Report Representation
Consolidated report in PDF format
Country Coverage
20+ Countries
Product Coverage
-
Dysport
-
Juvederm
-
Restylane
-
Radiesse
-
Profhilo
-
Rejuran
-
Juvelook
-
Xeomin
-
Botulax
-
Botox
Highlights of Report (Competitive & Revenue Landscape, by Product)
-
Product Revenues from 2018 to 2030
-
Brand Penetration in 20+ Countries
-
Product Market Share Evolution
-
Consumer Behavior Analysis
-
Strategic Initiatives from 2018 to 2024
-
SWOT Analysis by Manufacturers
Facial Injectables: Trends and Competitive Analysis Coverage Snapshot
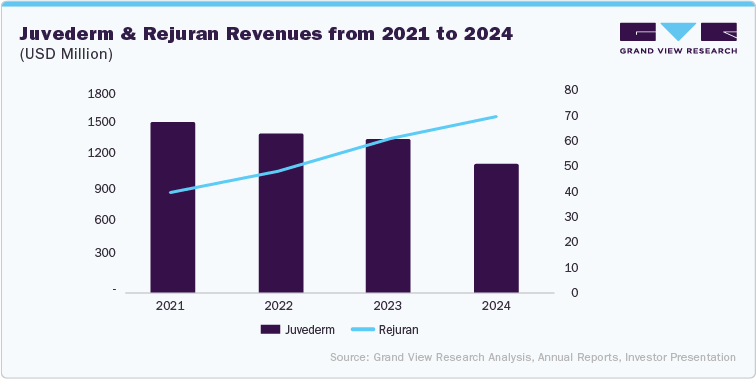
Recent trends indicate penetration of skin boosters in the facial injectable market. Moreover, established brands, such as Juvederm by Allergan, have seen a steady decline in their revenue from 2021, while brands, such as Rejuran by PharmaResearch Co., Ltd., have seen significant growth in the same period. Moreover, similar trends are seen across novel brands owing to superior technology, changing consumer sentiment from fillers to rejuvenation, and advanced marketing strategies by new players.
Some of the potential drawbacks highlighted by an aesthetic and oculoplastic surgeon in Beverly Hills in April 2024, “Hyaluronic acid fillers cause tissue expansion - it’s like a sponge attracting water. If too much filler is injected, it will result in the slow expansion of your natural tissue, stretching the skin and potentially accelerating the appearance of aging as the skin becomes less elastic. We now understand more about them and the potential negative consequences. This includes the knowledge that they can block lymphatics, causing swelling, but also the relatively new discovery that ‘while we initially thought that these products would only last between six to 12 months, we now know they can stay there for years.”
Critically, skin boosters can address volume issues and have become the physician’s choice for addressing rejuvenation and skin health. According to an aesthetic clinic, Belotero, a skin booster, helps in “deeply hydrating the skin, without creating unwanted volume.” Similarly, Profhilo, another skin booster primarily used in Europe, has seen a growing demand. An aesthetician commented, “Profhilo is great for those patients concerned with crepey skin that has lost its elasticity-it softens fine lines and helps plump up the skin.”
Neuromodulators, such as botulinum toxin, have maintained dominance in facial injectables. Moreover, novel brands are entering the developed markets of the U.S. & China, which are expected to cause significant changes in the forecast period. Medytox, Hugel, Daewoong, and Huons BioPharma, are some of the key South Korean manufacturers entering the two largest botulinum toxin markets in the U.S. and China.
Some of the key developments recorded in the facial injectable industry
Companies
Year
Month
Details
Daewoong Pharmaceutical
2024
June
Daewoong Pharmaceutical revealed that its global partner, Evolus, has launched Nuceiva in the Spanish market. Nuceiva, developed by Daewoong, is the same botulinum toxin (BTX) product marketed as Nabota in Korea and Jeuveau in North America
PharmaResearch Co., Ltd.
2024
May
Pharma Research obtained approval in South Korea for its botulinum toxin type A product, Reintox 100 units.
CGbio
2024
May
CGBio announced its entry into the European Aesthetic Market with its two injectable products: LUXX, a Polydioxanone (PDO) suture thread lift, and EGF skin booster
Merz Pharmaceutical GmbH
2024
February
Merz Pharmaceutical GmbH received approval for its botulinum toxin product Xeomin in China. The time from acceptance to approval was nearly 1,141 days. The product is indicated to treat severe canthus wrinkles (crow’s feet) in adults.
Medytox
2023
December
Medytox revealed that its subsidiary, NEWMECO, has introduced a new botulinum toxin product called NEWLUX. Approved by the Ministry of Food and Drug Safety in August, NEWLUX represents a next-generation formulation that eliminates animal-derived components from its raw material production.
Sinclair
2023
October
Sinclair, a subsidiary of Huadong Medicine Co., Ltd. & ATGC Co., Ltd., announced a strategic collaboration and licensing agreement through which Sinclair shall develop and commercialize ATGC’s BoNT-A, ATGC-110 for an aesthetic and therapeutic indication.
LG Chem
2023
October
LG Chem & BR Pharm signed a memorandum of understanding (MOU) to accelerate the former company’s skin booster, HP Vitaran, in the Chinese market.
CGbio
2023
October
CGBio signed a USD 100 million license agreement for its CaHA filler, FACETEM, for five years to penetrate the Chinese market.
CGBio
2023
September
CGBio announced that its CaHA filler, FACETEM, has been launched in Indonesia.
CGBio
2023
August
CGBio applied for a permit in Middle Eastern countries for its hyaluronic acid (HA) filler, GISELLELIGNE.
CGBio
2023
July
CGBio’s HA filler, Aileene received approval from the Australian government. Moreover, the company signed KRW 40 billion or USD 28.99 million with Australia’s aesthetic distributor, Amore Aesthetics.
CGBio
2023
June
CGBio signed a contract with a Chinese distributor, Shanghai Bijeong Trading LLC, worth KRW 70 billion or USD 50.51 million, allowing it to enter the Chinese hyaluronic acid market.
Allergan Aesthetics
2023
May
Allergan received U.S. FDA approval for its skin booster product, SKINVIVE, by JUVÉDERM. With this approval, the company became the first product to enter the skin booster paradigm in the United States.
Daewoong Pharmaceutical
2023
May
Daewoong Pharmaceutical of South Korea revealed to invest of 100 billion South Korean won (about USD 74.6 million) to construct a third manufacturing facility in Korea. This expansion aims to meet rising global demand for its botulinum toxin product, Nabota.
Share this report with your colleague or friend.
Pricing & Purchase Options
Service Guarantee
-
Insured Buying
This report has a service guarantee. We stand by our report quality.
-
Confidentiality
We are in compliance with GDPR & CCPA norms. All interactions are confidential.
-
Custom research service
Design an exclusive study to serve your research needs.
-
24/5 Research support
Get your queries resolved from an industry expert.
-
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


