- Home
- »
- Market Trend Reports
- »
-
Technological Trends And Innovations In Drug Delivery Devices
![Technological Trends And Innovations In Drug Delivery DevicesReport]()
Technological Trends And Innovations In Drug Delivery Devices
- Published: Aug, 2024
- Report ID: GVR-MT-100216
- Format: PDF, Horizon Databook
- No. of Pages/Datapoints: 50
- Report Coverage: 2024 - 2030
Report Overview
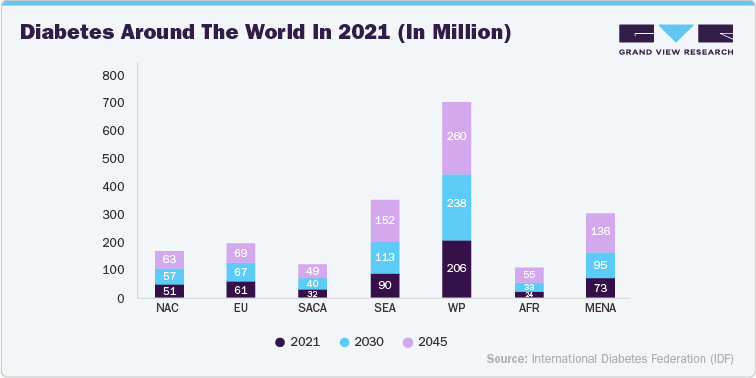
Technological advancements in drug delivery devices are driven by the need for better patient compliance, improved therapeutic outcomes, and personalized treatments. The growing prevalence of diabetes and cardiovascular diseases (CVD), coupled with the rise in biologic drug development, has spurred demand for advanced minimally invasive drug delivery methods. According to the International Diabetes Federation (IDF), the global diabetic population is expected to increase by 48% annually from 2017 to 2045, with a significant rise in diabetes cases in low and middle-income countries compared to developed nations. Additionally, the World Heart Federation highlights that adults with type 2 diabetes face a higher risk of developing CVD than non-diabetics.
This data drives U.S. manufacturers to focus on developing advanced minimally invasive drug delivery devices tailored to the needs of diabetic and CVD patients. By addressing these prevalent conditions with innovative solutions-such as continuous glucose monitors, smart insulin pens, and other advanced devices-manufacturers can improve patient outcomes and adherence. The regional distribution data also guides companies in targeting markets with higher diabetes prevalence, ensuring that new devices meet the specific needs of these populations and align with global health trends. The diagram below shows the regional distribution of the diabetic population.
Key Recent Trends In The Drug Delivery Systems
Medical manufacturing companies must continuously monitor significant trends in the drug delivery systems market. The following outlines the recent trends impacting the industry of injectable drug delivery systems.
Trends
Description
Challenges
Opportunities
Ultra Deep Freeze Drug Delivery Systems
Modern mRNA vaccines require storage at extremely low temperatures, down to -70°C, necessitating advanced packaging and materials that withstand these conditions.
Ensuring chemical stability and handling without interactions at ultra-low temperatures.
Silicone materials offer advantages and can withstand temperatures down to -90°C. Companies like Raumedic provide silicone components to enhance syringe systems.
Homecare Trend
Increasing preference for homecare therapy due to rising life expectancy and population growth, supported by medical device companies offering products for home use.
Adapting medical products for safe and effective use outside of hospital settings.
Digitization and modern sensor technology enable portable medical devices, allowing patients greater mobility and quality of life.
Wearable Drug Delivery Systems
Wearable devices, like insulin pumps, are gaining popularity, providing continuous and controlled drug administration for conditions such as diabetes.
Ensuring comfort, safety, and functionality in small, portable formats.
Development of aesthetically pleasing functional designs with integrated tubing and fluid paths. Smaller, less noticeable devices improve patient comfort and compliance.
Smart Drug Delivery Systems
Integration of smart technologies and digitization into drug delivery systems enhances functionality, user-friendliness, and data collection.
Incorporating advanced electronics and sensors into medical devices while maintaining safety and efficacy.
Smart components enable monitoring of vital functions and data evaluation, transforming standard devices into high-tech, data-conducting tools.
Sustainability in Drug Delivery Systems
Focus on sustainable practices in manufacturing, including use of natural ingredients, resource efficiency, and environmentally friendly packaging.
Balancing sustainability goals with product performance and regulatory compliance.
Emphasis on reducing emissions and resource use throughout production, supported by regulations like the Corporate Sustainability Reporting Directive (CSRD).
Innovations And Developments In Drug Delivery
Recent advancements in drug delivery systems have significantly transformed therapeutic approaches, particularly in managing chronic diseases like diabetes. Automated insulin delivery systems, such as Tandem Diabetes Care’s Mobi and Beta Bionics’ iLet ACE, exemplify innovations that enhance patient autonomy and treatment efficacy. Additionally, smart insulin pens like Biocorp’s Mallya enable real-time data tracking for better management. The drivers of these innovations include the increasing prevalence of chronic diseases, advancements in technology enabling personalized medicine, and a growing emphasis on patient-centric healthcare solutions. Regulatory support and collaboration between tech companies and healthcare providers also foster rapid development in this field.
Innovation
Description
Male Birth Control Gel
Nestorone gel, a reversible contraceptive for men, is applied to the upper arms and shoulders for absorption through the skin.
Halozyme’s Enhanze Technology
Utilizes RHuPH20 enzyme to enhance the dispersion and absorption of injected therapeutic drugs.
Nemaura’s 48-hour Transdermal Pain Patch
Employs Micro-Patch technology to administer solid dose therapeutics for extended pain relief over 48 hours.
Microbubble Technology
Uses microbubbles and ultrasound to deliver drugs across the blood-brain barrier effectively.
Intranasal Antipsychotic
INP105 nasal spray contains olanzapine for rapid tranquilization of patients experiencing acute agitation.
Kapspargo Sprinkle
An extended-release heart medication by Sun Pharma, in capsule form, designed to be sprinkled onto soft foods.
Milk-derived Exosome Platform
Developed by Roche and PureTech, this technology uses milk-derived exosomes for the oral administration of complex drugs.
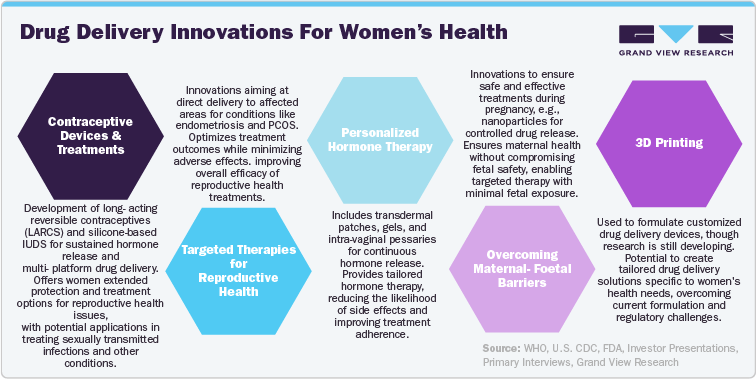
Drug Delivery Innovations For Women’s Health
From nanoparticles to transdermal patches, pharmaceutical technology explores the newest drug delivery technologies for women's health. These innovations are revolutionizing contraceptive methods and improving treatments for reproductive health issues, leading to more personalized and effective healthcare. Women's unique microenvironments, such as the vagina and placenta, necessitate specific design considerations for optimal drug delivery. Additionally, delivering multiple therapies via a single device requires careful balancing of biocompatible materials to ensure safety and efficacy. Notably, only 3.7% of clinical trials from 2007 to 2020 focused on female gynecology, highlighting a disparity in preclinical funding and the 'disease burden' between women-specific and men-specific disorders. This limited focus underscores the need for increased investment and research to develop and implement effective drug delivery technologies for women's health. Here are some recent innovations in this field that could significantly impact the future.
Emerging Trend: Oral Dosage Forms
Another emerging trend in drug delivery is the use of oral dosage forms, which is considered the most natural, safe, and convenient method for administering medication. In the U.S., the majority of drugs are taken orally due to the substantial systemic effects they produce after being absorbed through various parts of the gastrointestinal tract (GIT).
Advancements In Drug Release Technologies For Oral Dosage Forms
Recent technological strides aim to enhance drug release from oral dosage forms. Nanoparticles, both organic and inorganic, have emerged as promising tools in drug delivery. They improve drug tolerability, pharmacological specificity, targeting, and biodegradability. Numerous nanocarriers have been incorporated into oral drug formulations, offering advantages such as protection from harsh gastrointestinal conditions, improved systemic absorption, precise targeting, and controlled drug release.
Enhancing Drug Absorption: Innovations And Strategies
Various strategies are being explored to boost drug absorption in the gastrointestinal tract (GIT), including the use of permeation enhancers, nanosized carriers, and drug structure modifications. Permeation enhancers can enhance drug absorption by improving membrane permeability rather than simply increasing solubility. Additionally, advanced microfabricated devices, created using techniques like microfabrication and 3D printing, are being developed to provide precise control over drug delivery and discover new interactions between materials and cells. For example, silicon nanowires on microbeads have been shown to increase adhesion to epithelial cells and extend GIT residence time significantly.
Another innovative approach involves a "robotic pill" featuring an enteric-coated capsule containing a folded poly(ethylene) balloon and a dissolvable needle made from PEG. Once released in the intestine, the balloon inflates and pierces the intestinal wall, facilitating extended drug release and absorption.
Impact On Patient Compliance And Experience
Innovations designed to enhance oral drug absorption and control release technology are crucial for improving patient compliance and the overall treatment experience. Oral drug delivery is favored for its ease of use, painlessness, cost-effectiveness, and high patient compliance. However, limited efficacy of some oral medicines may lead to a preference for alternative routes, potentially affecting patient compliance. Age-related factors also influence the choice of dosage form, with liquid forms being more suitable for young children. Flavored liquids may aid in medication adherence among younger patients.
Challenges And Considerations In Development
Pharmaceutical companies face several challenges in developing innovative oral dosage forms, such as biochemical and physical barriers in the GIT that can restrict drug absorption. Macromolecular drugs are susceptible to denaturation and instability in the stomach and intestine. Although excipients and technologies like nanoparticles have shown high efficacy in preclinical models, they often face hurdles in clinical trials. Common permeation enhancers have achieved low oral availability rates, and complex manufacturing processes, as seen with the "robotic pill," present additional difficulties.
Case Studies: Success Stories In Oral Dosage Form Innovation
Research into improving medicinal products through combining multiple active substances has shown potential benefits. Fixed-dose combination (FDC) formulations, which use complementary mechanisms of multiple substances, can enhance drug efficacy, reduce side effects, and improve patient compliance. These combinations often require lower doses of each drug and simplify dosing schedules. Below mention are some of the examples of case studies:
Pediatric Drug Delivery Case Study
Objective: To develop a suitable and palatable oral pediatric formulation for children aged three months to 12 years.
Challenges:
1. Dosage Forms: Young children may struggle with tablets and capsules, requiring alternative forms such as liquid suspensions, granules, or mini tablets.
2 Taste Masking: Ensuring the formulation is palatable is crucial for compliance.
3. Excipients: Special care is needed to choose excipients appropriate for pediatric use, which may differ from those used in adult formulations.
Study Approach:
1. Taste Assessment: Quotient Sciences conducted a study to evaluate various flavor and sweetener combinations in a liquid suspension. Healthy volunteers tasted these formulations using a sip-and-spit method and rated them on various taste characteristics.
2. Bioavailability Testing: The optimal formulation from the taste assessment was tested for bioavailability compared to a reference adult tablet formulation.
Outcome: The integrated approach of formulation development, taste masking, and bioavailability testing allowed the client to efficiently create a palatable pediatric drug product and enter clinical trials successfully.
Conclusion: Integrating drug substance development, clinical testing, and real-time manufacturing speeds up the process of bringing pediatric formulations to market. This approach is beneficial for accelerating complex drug development from initial trials to commercial launch.
Solubility-Enhancement Case Study
Objective: To address poor aqueous solubility of a new chemical entity (NCE) which led to low oral exposure and variability in early clinical trials.
Challenges: The NCE exhibited poor solubility, non-linear pharmacokinetics (PK), high variability, and a significant food effect, hindering progression to patient studies.
Approach: Quotient Sciences used an integrated Translational Pharmaceutics approach to enhance solubility by developing and screening three solubility-enhancing formulations:
1. Micronized Formulation: Reduced particle size of the API.
2. Self-emulsifying Drug Delivery System: Utilized a lipid-based formulation.
3. Amorphous Formulation: Applied spray-dried dispersion (SDD).
Process:
1. Part One: Small-scale drug products were tested in healthy volunteers using a six-period crossover design to compare human PK data.
2. Part Two: Higher doses were administered to establish safety margins and determine dose linearity.
Outcome: A lead formulation was identified within six months, overcoming solubility issues and allowing the compound to advance to patient clinical studies efficiently.
Future Trends in Oral Drug Delivery Systems
The future of oral dosage forms and drug release technology holds promise with ongoing advancements aimed at improving drug absorption and extending release times. Innovations like the "robotic pill" could revolutionize oral drug delivery by using microneedles to enhance drug permeation and absorption. Continued research and development are essential to overcoming current challenges and advancing these technologies from proof-of-concept to clinical application.
Share this report with your colleague or friend.
GET A FREE SAMPLE
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities.
Contact us now to get our best pricing.
![esomar icon]()
ESOMAR certified & member
![ISO]()
ISO Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


