Report Overview
Cell therapies have emerged as a leading sector in the biopharmaceutical industry, marked by significant advancements toward potentially curative treatments for complex diseases using innovative scientific approaches. This field has recently seen a surge in billion-dollar investments and numerous record-breaking approvals, highlighting its status as one of the most dynamic and promising areas within the biopharmaceutical industry. A few recent instances in this regard are as follows.
-
In March 2024, China’s National Medical Products Administration (NMPA) approved CARsgen's autologous CAR T-cell therapy, zevorcabtagene autoleucel, for treating adult patients with relapsed/refractory multiple myeloma who have progressed after at least three lines of treatment, including a proteasome inhibitor and an immunomodulatory drug
-
In February 2024, The Food and Drug Administration permitted an accelerated approval to Iovance Biotherapeutics lifileucel, a tumor-derived autologous T cell immunotherapy. The approved therapy is for adult patients with unresectable or metastatic melanoma previously treated with a PD-1 blocking antibody, and if BRAF V600 positive, a BRAF inhibitor with or without a MEK inhibitor
As more cell therapies gain commercial approval in the United States and other regions, biopharmaceutical companies and contract development and manufacturing organizations (CDMOs) adapt to support clinical and commercial production.
Alongside the rapid rise in investigational new drug applications submitted to the FDA, the demand for commercial CAR-T cell therapies continues to exceed the available supply.
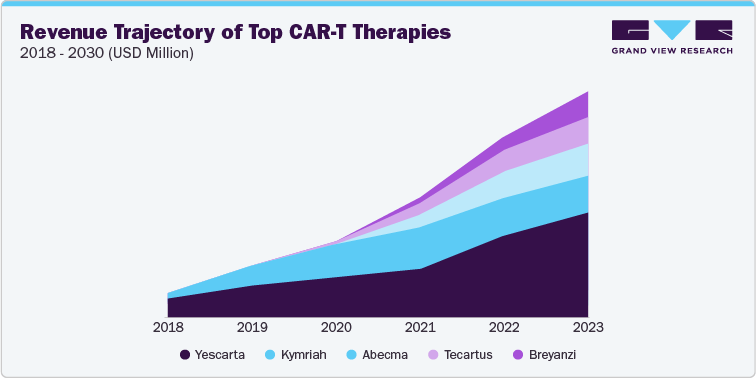
Kite Pharma's CAR T-cell therapy Yescarta dominated the list of best-selling cell therapies and generated USD 1.49 billion in revenue in 2023. To meet the rising demand for this therapy, Kite is expanding its manufacturing network capacity. Moreover, The FDA aims to review and approve 10-20 gene and cell therapies annually by 2025. As more products receive regulatory approval, the cell therapy market size is projected to reach $20.07 billion by 2030.
However, despite the steady stream of approvals and promising growth, the high manufacturing costs of these therapies often make them inaccessible to most patients. Companies are working to streamline and optimize the complex and labor-intensive cell therapy manufacturing process to tackle this issue.
Cell Therapy: Approved Products And Pipeline Analysis Report Scope
|
Report Attribute |
Details |
|
Cell Therapy-approved Products: Deep Drive Analysis: |
|
|
Cell Therapy Clinical Trials Landscape |
|
There is a rising number of clinical studies focusing on developing cellular therapies. This surge reflects a growing recognition of the potential of cellular therapies across a spectrum of medical conditions and highlights the increasing focus of pharmaceutical companies to explore & harness the therapeutic potential of cells. For instance, According to The American Society of Gene & Cell Therapy (ASGCT), the number of therapies at all three phases of clinical pipeline development has increased since the end of 2023, where Phase I therapy particularly saw increased growth.
One significant factor fueling the high number of clinical studies is the promising outcomes observed in early-phase trials. Positive results from initial studies have instilled confidence in the efficacy and safety of cellular therapies, encouraging further exploration & investment. Conditions such as cancer, autoimmune disorders, and degenerative diseases are being targeted, with researchers aiming to unlock the full therapeutic potential of various cell types, including stem cells & immune cells.
Top Therapeutic Areas Under Investigation - Cancer indications continue to dominate the pipeline
Some of the key areas of cell therapy clinical trials include oncology, autoimmune diseases, and neurological disorders, among others. In oncology, CAR-T cell therapy trials target various hematologic malignancies, such as leukemia, lymphoma, and multiple myeloma, with some trials also exploring the potential of CAR-T for solid tumors. In addition, TIL therapy trials focus on melanoma, cervical cancer, and other solid tumors.
For neurological disorders, several studies are investigating stem cell therapies for conditions like spinal cord injuries, Parkinson’s disease, and stroke recovery. Other significant indications for cell therapy trials include cardiovascular diseases, musculoskeletal disorders, and genetic disorders. Some trials explore the use of stem cells to repair heart tissue after myocardial infarction, while others use mesenchymal stem cells (MSCs) for treating osteoarthritis, cartilage repair, and bone regeneration. The top three non-oncology indications in the pipeline were acute spinal cord injury, respiratory distress syndrome, and graft-versus-host disease.
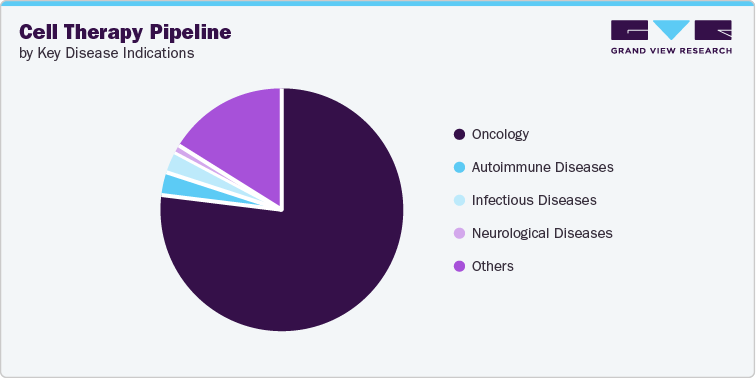
The oncology segment remained the top area of cell therapy development. Cell therapy is an innovative and rapidly evolving field in cancer research that involves using live cells to treat, prevent, or cure diseases, including various forms of cancer. CAR-T therapies have shown remarkable success in treating certain types of blood cancers like acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL). According to ASCGT, the top three oncology indications in the pipeline were liver cancer, acute myelogenous leukemia, and pancreatic cancer.
Moreover, stem cell therapies, such as hematopoietic stem cell transplantation (HSCT), are well-established for blood cancers like leukemia and lymphoma. Emerging research is investigating the potential of other types of stem cells, such as mesenchymal stem cells, in cancer therapy.





