
Thyroid Function Testing Market Size, Share & Trends Analysis Report By Test Type (TSH Test, T4 Test, T3 Test, Free T4 Test), By End-use (Clinics, Diagnostic Laboratories, Hospitals, Research Laboratories & Institutes), By Region, And Segment Forecasts, 2024 - 2030
- Report ID: GVR-4-68040-236-4
- Number of Report Pages: 125
- Format: PDF
- Historical Range: 2018 - 2022
- Forecast Period: 2024 - 2030
- Industry: Healthcare
Thyroid Function Testing Market Trends
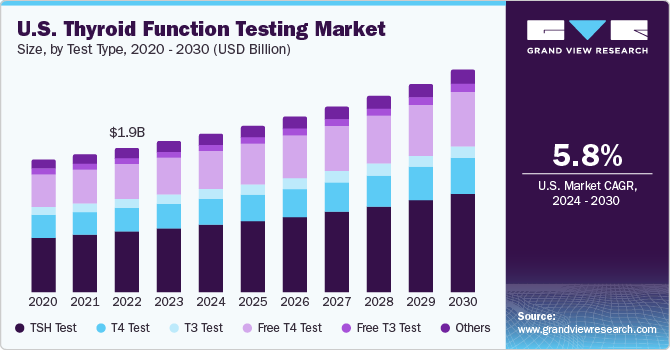
The global thyroid function testing market size was estimated at USD 4.79 billion in 2023 and is expected to grow at a CAGR of 6.31% from 2024 to 2030. The market growth is attributed to the increasing incidence of thyroid disorders, growing awareness, rising number of screening programs, and high healthcare expenditures are the major factors driving market growth. Furthermore, increasing the adoption of thyroid function tests in pregnancy will further offer lucrative growth opportunities. Increasing awareness about the treatment and diagnosis of thyroid cancer is the major factor driving market growth. For instance, Thyroid Awareness Month in January shines a spotlight on the prevalence of thyroid-related diseases and thyroid cancer, emphasizing the importance of early detection and treatment.
According to the CDC, thyroid disease is prevalent, particularly among older individuals & women, highlighting the need for thorough thyroid evaluations by experienced medical professionals. Statistics reveal that approximately 1 in 10 people suffer from a thyroid disorder, with at least 1 in 8 women developing a thyroid disorder in their lifetime. Moreover, International Thyroid Awareness Week (ITAW) and World Thyroid Day 2023, Merck, together with the Executive Board of the Indonesian Medical Association ("PB IDI"), and the Central Board of the Indonesian Thyroid Association ("PP InaTA") signed a Memorandum of Understanding to launch the Thyroid RAISE program.
This initiative signifies a joint commitment to enhancing healthcare professionals' capabilities and raising public awareness regarding the importance of screening, diagnosing, and treating thyroid disorders in high-risk adult populations. In addition, the program aims to improve screening for congenital hypothyroidism (SHK) in newborns, as well as the treatment of hyperthyroidism and hypothyroidism in Indonesia. The Thyroid RAISE program serves as a significant step towards improving thyroid healthcare in Indonesia. By increasing awareness, enhancing healthcare professional capabilities, and ensuring access to quality healthcare services, the program aims to reduce the burden of thyroid disorders and improve the health outcomes of individuals affected by these conditions.
In addition, hypothyroidism affects an estimated 12.4 million individuals, with a very low treatment rate of only 1.9%. This condition can be inherited from mothers to their children, leading to congenital hypothyroidism in newborns, which can result in severe health issues and intellectual disabilities. Similarly, hyperthyroidism affects around 13.2 million individuals, with a treatment rate of only 6.2%. Addressing these alarming statistics, it is imperative to enhance the capabilities of healthcare practitioners, especially doctors across all disciplines, in screening & diagnosing thyroid disorders early. Early detection is crucial in preventing further complications and serious health problems associated with thyroid disorders.
Furthermore, The Up To Here campaign, launched by the American Association of Clinical Endocrinologists (AACE) in January 2020, aims to raise awareness of thyroid diseases among Americans. The campaign seeks to educate the public about recognizing symptoms, understanding risk factors, and the importance of seeking treatment. Technological advancements have significantly transformed the assessment of thyroid function, moving from basic clinical observations to a diverse range of advanced testing methods. The current landscape of thyroid health research is characterized by a variety of laboratory techniques. It becomes evident that advancements in immunoassays, molecular testing, automation, and the exploration of innovative biomarkers are shaping the present and future landscape of thyroid diagnostics. These technological advancements are expected to create growth opportunities in the coming years.
Market Concentration & Characteristics
The market exhibits a moderate degree of innovation, primarily driven by advancements in technology and a growing understanding of thyroid disorders. These innovations have led to the development of more accurate and efficient testing methods, including immunoassays, molecular testing, and automation. In addition, the exploration of novel biomarkers has opened up new avenues for diagnosing thyroid disorders. These advancements have not only improved the accuracy and reliability of thyroid function testing but have also enhanced the overall efficiency of thyroid diagnostics
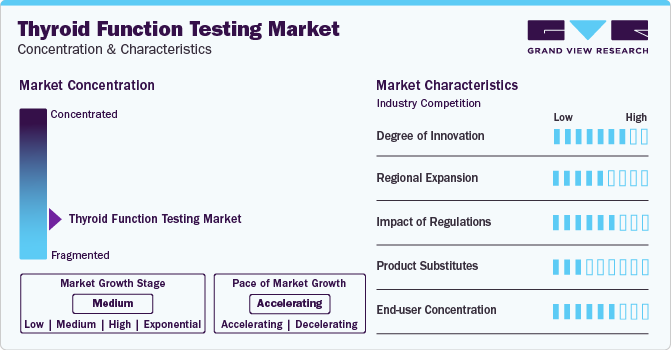
The thyroid function testing market has seen a moderate degree of regional expansion activity, with companies seeking to expand their product portfolios and geographic presence. For instance, in May 2022, Thyrocare Technologies Ltd., India's first advanced fully-automated laboratory chain, opened its first Regional Processing Lab (RPL) in Nagpur. This new lab marks Thyrocare's 17th such facility in India, showcasing the company's commitment to expanding its network and enhancing its diagnostic capabilities
Regulatory measures greatly impact the market, especially for diagnostic labs. Compliance with standards and regulations ensures the quality and accuracy of tests. For instance, the Clinical Laboratory Improvement Amendments (CLIA) in the U.S. set standards for lab testing to ensure quality and accuracy, affecting how labs operate and the tests they offer
In this market, there are a few direct substitutes for traditional lab-based tests, given the complexity and specificity of thyroid function evaluations. However, indirect substitutes could include imaging techniques like ultrasound or functional medicine approaches. These alternatives are often used in conjunction with, rather than as a replacement for, standard thyroid function tests
End-user concentration in this market is notable, with diagnostic laboratories and hospitals being the primary end-users. For example, Thyrocare Technologies Ltd. offers a range of thyroid function tests and serves as a key player in this market, catering to the growing demand for accurate thyroid diagnostics
Test Type Insights
The TSH test segment led the market and accounted for a share of 41.32% in 2023 and is anticipated to grow at the fastest CAGR from 2024 to 2030. TSH tests are the recommended initial screening for thyroid dysfunction, utilizing highly sensitive third-generation immunometric assays since the late 1980s, capable of detecting TSH levels <0.01 mIU/L. Globally, about 2 billion people are at risk of iodine deficiency, a precursor to thyroid disease. An estimated 200 million people worldwide have some form of thyroid disease, with women being affected more than men at a ratio of 8:1. The importance of TSH testing is a major driving factor for the use of TSH tests. In addition, the launch of technologically advanced products will further drive the segment growth.
For instance, in November 2021, Bloom Diagnostics launched the Bloom Thyroid Test, a test that helps in detecting hypothyroidism. It is a single-use kit and can be used to test adults for TSH as a means of detecting thyroid dysfunction in adults. The Bloom Thyroid Test requests users to provide details about their medication use, including medications like Lithium or Amiodarone, known to affect thyroid function. In addition, the test takes into account the pregnancy status of women, whether they have recently given birth or plan to conceive soon. This is important because thyroid hormone production increases during pregnancy, rendering TSH tests unreliable during this period. The free T4 test is anticipated to grow at a significant CAGR over the forecast period.
The free thyroxine (FT4) test is crucial in assessing thyroid function, as it measures the amount of unbound T4 hormone in the blood, which is the active form of thyroxine. This test is essential for diagnosing and monitoring thyroid disorders, such as hyperthyroidism and hypothyroidism. Factors driving the segment growth include the increasing prevalence of thyroid disorders globally, rising awareness about the importance of early detection and management of thyroid diseases, and rising adoption of advanced diagnostic technologies. In addition, factors, such as the growing aging population, which is more prone to thyroid disorders, and increasing focus on personalized medicine are driving the demand for FT4 testing.
End-use Insights
The hospitals segment led the market and accounted for a share of 37.29% in 2023. Thyroid function testing plays a pivotal role in hospital settings for diagnosing and managing various thyroid disorders. Endocrinologists and primary care physicians often rely on these tests to assess thyroid hormone levels accurately, guiding treatment decisions for conditions like hyperthyroidism, hypothyroidism, and thyroid nodules. Moreover, thyroid function tests are essential in preoperative evaluations and monitoring patients undergoing thyroid hormone replacement therapy or thyroid surgery. Factors driving the segment growth include the increasing prevalence of thyroid disorders globally, rising awareness among healthcare professionals about the importance of early diagnosis, and advancements in diagnostic technologies facilitating quicker and more accurate test results.
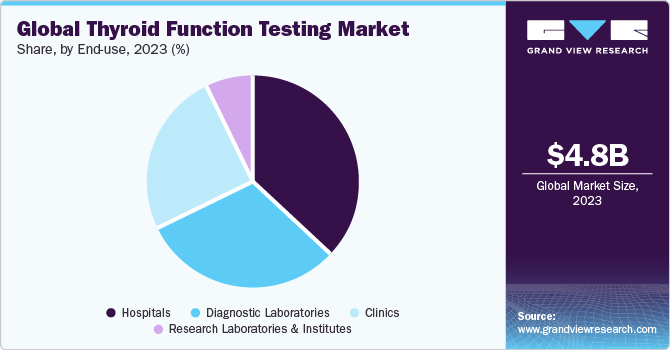
In addition, the growing geriatric population and improving healthcare infrastructure in developing regions contribute to the market growth. In addition, the diagnostic laboratories segment is anticipated to grow at the fastest CAGR from 2024 to 2030. Diagnostic laboratories play a crucial role in thyroid function testing, offering a wide range of tests to assess thyroid health. One trend in this sector is the increasing adoption of automated platforms and immunoassay techniques, which provide faster and more accurate results. Another trend is the integration of thyroid function tests into comprehensive wellness panels, allowing for more comprehensive health assessments.
Driving factors for thyroid function testing in diagnostic laboratories include the rising prevalence of thyroid disorders, growing awareness among individuals about the importance of thyroid health, and increasing demand for preventive healthcare. In addition, advancements in technology, such as the development of novel biomarkers and point-of-care testing devices, are driving market growth. Examples of diagnostic laboratories offering thyroid function tests include Quest Diagnostics, LabCorp, and Thyrocare Technologies Limited.
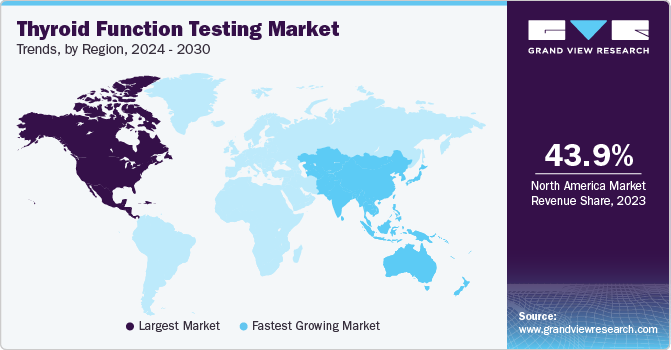
Regional Insights
North America accounted for a share of 43.92% in 2023 owing to the high prevalence of thyroid disorders in the region, driven by factors, such as iodine deficiency, autoimmune diseases, and lifestyle changes. Thyroid conditions are prevalent in the United States, with more than 12% of the population expected to develop a thyroid condition during their lifetime. An estimated 20 million Americans are currently affected by some form of thyroid disease. Alarmingly, up to 60% of individuals with thyroid disease are unaware of their condition, highlighting the need for regular thyroid function testing and raising thyroid health awareness.
U.S. Thyroid Function Testing Market Trends
The U.S. thyroid function testing market is expected to grow significantly over the forecast period due to the preference for point-of-care testing devices, allowing for convenient and rapid thyroid function assessments. Examples include the inclusion of thyroid tests in wellness packages offered by major diagnostic laboratories like Quest Diagnostics and the development of portable thyroid testing devices by companies like Abbott Laboratories.
Europe Thyroid Function Testing Market Trends
The thyroid function testing market in Europe held a significant revenue share in 2023. Thyroid diseases are prevalent among endocrine disorders in Europe, with approximately 100 million patients. Thyroid Stimulating Hormone (TSH) is the primary protein marker used by clinicians for diagnosis. The European thyroid function testing market is dynamic, driven by technological advancements, rising awareness, and a growing geriatric population. Government initiatives and patient preference for non-invasive tests also contribute to market growth.
The UK thyroid function testing market is expected to grow over the forecast period due to a shift towards point-of-care testing for thyroid disorders, driven by the need for quick results and convenience. There is also a growing emphasis on personalized medicine, leading to the development of more targeted diagnostic approaches.
The thyroid function testing market in France is expected to grow considerably over the forecast period due to a rise in demand for advanced diagnostic techniques, including molecular testing, to improve the accuracy of thyroid disorder diagnosis. There is also a growing focus on integrating digital health technologies for enhanced patient management.
The Germany thyroid function testing market is expected to register a significant CAGR from 2024 to 2030 due to the rising adoption of automated and high-throughput testing methods, enhancing efficiency and reducing turnaround times. There is also a trend towards decentralized testing to improve access and convenience for patients.
Asia Pacific Thyroid Function Testing Market Trends
The thyroid function testing market in Asia Pacific is anticipated to witness the fastest CAGR from 2024 to 2030, driven by factors, such as increasing prevalence of thyroid disorders, particularly in countries like India and China. There is a rising demand for cost-effective and accurate diagnostic tests, leading to the adoption of point-of-care testing solutions. In addition, the market is influenced by improving healthcare infrastructure, growing awareness about thyroid health, and presence of key market players expanding their operations in the region. The regional market is poised for substantial growth and presents lucrative opportunities for industry players.
The China thyroid function testing market is expected to grow considerably over the forecast period due to the growing focus on improving healthcare R&D aided by the development of novel technologies.
The thyroid function testing market in Japan is expected to witness growth over the forecast period on account of the presence of a well-established healthcare system.
Latin America Thyroid Function Testing Market Trends
The Latin America thyroid function testing market was a lucrative regional market. Technological advancements in the region are anticipated to fuel market growth.
The thyroid function testing market in Brazil is expected to grow over due to the increasing awareness about thyroid disorders, improving healthcare infrastructure, and rising healthcare expenditure.
Middle East & Africa Thyroid Function Testing Market Trends
The Middle East & Africa thyroid function testing market was another lucrative regional market due to the advanced diagnostic techniques, including molecular testing, to enhance the accuracy of diagnosis. In addition, it is characterized by the presence of both domestic and international companies competing to offer innovative testing solutions.
The thyroid function testing market in Saudi Arabia is expected to grow over the forecast period owing to the presence of key players strengthening their presence in the region and offering innovative testing solutions.
Key Thyroid Function Testing Company Insights
Some of the leading players operating in the thyroid function testing market include Quest Diagnostics, Laboratory Corporation of America Holdings, and Ulta Lab Tests, LLC. Key players are using existing customer bases in the region to prioritize maintaining high-quality standards and gain high market size access. This strategy is useful for brands that have already built trust in the market. These players are heavily investing in advanced technology and infrastructure, allowing them to process & analyze a large volume of samples efficiently. Moreover, companies undertake various strategic initiatives with other companies and distributors to strengthen their market presence.
ZRT Laboratory, Paloma, and LifeLabs are some of the emerging market participants. These companies focus on expanding their test menu, forming partnerships, and investing in technological advancements.
Key Thyroid Function Testing Companies:
The following are the leading companies in the thyroid function testing market. These companies collectively hold the largest market share and dictate industry trends.
- Quest Diagnostics
- Laboratory Corporation of America Holdings
- Ulta Lab Tests, LLC
- DHA Laboratory
- Everlywell, Inc
- ZRT Laboratory
- Paloma
- LifeLabs
- Neuberg Diagnostics Pvt. Ltd.
- Thyrocare
Recent Developments
-
In March 2023, Everly Health introduced a new virtual care program that combines lab testing with telehealth consultations to assess various health conditions, such as COVID-19, flu, sexually transmitted infections (STIs), thyroid issues, weight management, and men's and women's health
-
In September 2023, Neuberg Diagnostics completed the merger of Supratech and Anand Reference Laboratory. This merger represents a major milestone in the quest to offer unmatched healthcare solutions. By combining these laboratories, the company strengthens its dedication to delivering exceptional diagnostics and patient care
Thyroid Function Testing Market Report Scope
|
Report Attribute |
Details |
|
Market size value in 2024 |
USD 5.04 billion |
|
Revenue forecast in 2030 |
USD 7.27 billion |
|
Growth rate |
CAGR of 6.31% from 2024 to 2030 |
|
Actual data |
2018 - 2022 |
|
Forecast period |
2024 - 2030 |
|
Quantitative units |
Revenue in USD million and CAGR from 2024 to 2030 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Test type, end-use, and region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
|
Country scope |
U.S.; Canada; Germany; UK; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; South Korea; Thailand; Australia; Brazil; Mexico; Argentina; South Africa; UAE; Kuwait; Saudi Arabia |
|
Key companies profiled |
Quest Diagnostics; Laboratory Corporation of America Holdings; Ulta Lab Tests, LLC; DHA Laboratory; Everlywell, Inc.; ZRT Laboratory; Paloma; LifeLabs; Neuberg Diagnostics Pvt. Ltd.; Thyrocare |
|
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. Explore purchase options |
Global Thyroid Function Testing Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global thyroid function testing market report based on test type, end-use, and region:
-
Test Type Outlook (Revenue, USD Million, 2018 - 2030)
-
TSH Test
-
T4 Test
-
T3 Test
-
Free T4 Test
-
Free T3 Test
-
Others
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Clinics
-
Diagnostic Laboratories
-
Hospitals
-
Research Laboratories & Institutes
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Mexico
-
Argentina
-
-
Middle East & Africa (MEA)
-
South Africa
-
Saudi Arabia
-
Kuwait
-
UAE
-
-
Frequently Asked Questions About This Report
b. The global thyroid function testing market size was estimated at USD 4.79 billion in 2023 and is expected to reach USD 5.04 billion in 2024.
b. The global thyroid function testing market is expected to grow at a compound annual growth rate of 6.31% from 2024 to 2030 to reach USD 7.27 billion by 2030.
b. North America dominated the thyroid function testing market with a share of 47.74% in 2023, driven by afactors such as iodine deficiency, autoimmune diseases, and lifestyle changes. Additionally, the availability of advanced healthcare infrastructure and diagnostic technologies in North America is driving market growth. The region's focus on preventive healthcare and early disease detection also contributes to the expansion of the thyroid function testing market in North America.
b. Some of the major companies in the thyroid function testing market includeQuest Diagnostics, Laboratory Corporation of America Holdings, Ulta Lab Tests, LLC, DHA Laboratory, Everlywell, Inc, ZRT Laboratory, Paloma, LifeLabs, Neuberg Diagnostics Pvt. Ltd and Thyrocare
b. Key factors that are driving the thyroid function testing market growth include increasing incidence of thyroid disorders, growing awareness and screening programs and increased healthcare expenditure are the major driving the growth of the market. Furthermore, increasing adoption of thyroid function tests in pregnancy will further offer lucrative opportunities during the review period.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."




