- Home
- »
- Medical Devices
- »
-
Polynucleotides Injectable Market Size, Industry Report, 2030GVR Report cover
![Polynucleotides Injectable Market Size, Share & Trends Report]()
Polynucleotides Injectable Market (2025 - 2030) Size, Share & Trends Analysis Report By Application (Eyes, Lips, Forehead, Jawline & Cheekbones), By End-use (MedSpas, Aesthetic & Cosmetic Centers), By Region, And Segment Forecasts
- Report ID: GVR-4-68040-361-6
- Number of Report Pages: 150
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Polynucleotides Injectable Market Summary
The global polynucleotides injectables market size was estimated at USD 127.4 million in 2024 and is projected to reach USD 293.4 million by 2030, growing at a CAGR of 15.1% from 2025 to 2030. Technological advancements in biotechnology primarily drive market growth, as do the increasing demand for skin rejuvenation, personalized medicine, and investments in research and development (R&D).
Key Market Trends & Insights
- In terms of region, Asia Pacific was the largest revenue generating market in 2024.
- Country-wise, Thailand is expected to register the highest CAGR from 2025 to 2030.
- In terms of segment, eyes accounted for a revenue of USD 92.9 million in 2024.
- Forehead is the most lucrative application segment, registering the fastest growth during the forecast period.
Market Size & Forecast
- 2024 Market Size: USD 127.4 Million
- 2030 Projected Market Size: USD 293.4 Million
- CAGR (2025-2030): 15.1%
- Asia Pacific: Largest market in 2024
According to an article published by PMTA Journal in March 2024, polynucleotide injections gained prominence in aesthetic medicine due to their beneficial effects on skin, hair, and other tissues, as explored in this comprehensive review.

The growing demand for advanced skin rejuvenation treatments is a primary driver of the polynucleotides injectable industry. As individuals seek more effective solutions to address signs of aging, such as fine lines, wrinkles, and skin laxity, the interest in novel injectable treatments such as polynucleotides has surged. For instance, a recent study published in the journal Dermatologic Therapy found that polynucleotide injections improved skin tone, fine lines, skin elasticity, and hydration in patients in their 30s and 40s.
The increasing focus on personalized medicine also contributed to the growth of the market. As healthcare providers and patients alike seek customized approaches to skin rejuvenation, polynucleotides' ability to stimulate the body's natural collagen production and cellular regeneration has made them a popular choice. This trend is particularly evident in the rising demand for personalized aesthetic treatments, which is expected to continue expanding the market.
Finally, integrating innovative technologies, such as digital imaging and automated injection systems, has further propelled the polynucleotides injectable industry. These advancements improved polynucleotide injections' precision, consistency, and safety, making them more appealing to healthcare providers and patients. As the aesthetic industry continues to evolve, incorporating cutting-edge technologies is expected to remain a vital driver of the polynucleotide injectable industry. For instance, in 2022, Pulse Light Clinic in London is introducing PhilArt by Croma, an innovative polynucleotide injection treatment recognized for significantly enhancing skin hydration, boosting elasticity, and stimulating natural collagen production - a pioneering advancement in skin rejuvenation.
Market Concentration & Characteristics
The polynucleotides injectable industry is experiencing notable innovations as companies focus on enhancing formulation technologies, integrating bioactive components, and utilizing advanced delivery systems. These advancements improve product efficacy, ensure superior biocompatibility, and enhance treatment outcomes. The introduction of next-generation polynucleotide-based injectables offers improved skin regeneration, prolonged therapeutic effects, and better patient satisfaction. In addition, innovative delivery mechanisms and combination formulations are setting new standards in minimally invasive aesthetic and therapeutic procedures, providing effective solutions for skin rejuvenation, wound healing, and tissue repair with long-lasting results.
Leading manufacturers in the polynucleotides injectable industry, such as PHARMA RESEARCH, BIOPLUS CO., LTD., and AMEELA, are strengthening their positions through strategic mergers and acquisitions. These companies actively pursue initiatives such as developing innovative product portfolios, forming collaborative partnerships, and expanding their reach into emerging markets. For instance, In January 2024, Amedica collaborated with Acquisition Aesthetics Training Academy to offer 2024 training courses on Ameela Polynucleotides. These programs will focus on the science, treatment techniques, safety, and achieving optimal results with polynucleotide injectables.
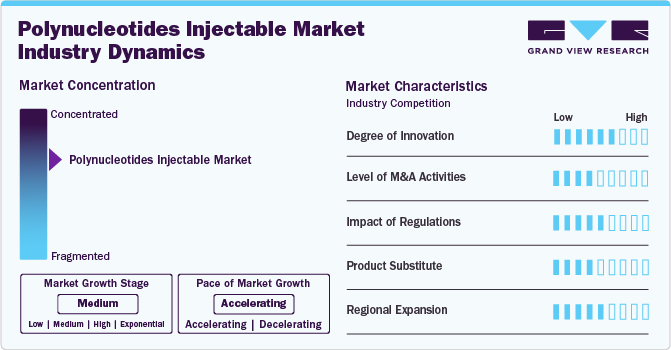
Regulatory frameworks play a key role in the polynucleotides injectable industry by establishing essential safety, quality, and efficacy standards. While stringent regulations may extend the approval timelines for new products, potentially delaying their market launch, they are critical to ensuring patient safety and product reliability. These frameworks guarantee that only high-quality, safe, and biocompatible polynucleotide injectables reach the market, protecting patients from substandard formulations and ensuring effective outcomes in aesthetic and therapeutic applications.
There are direct substitutes for polynucleotide injectables in various applications. Moreover, in certain aesthetic or therapeutic contexts, alternative treatments such as hyaluronic acid fillers, collagen stimulators, or platelet-rich plasma (PRP) therapies may be considered based on individual patient needs and clinical goals. While these alternatives can address some aspects of skin rejuvenation, wound healing, or tissue repair, they do not fully replicate polynucleotide injectables' unique regenerative properties and long-lasting effects.
Key players in the polynucleotides injectable industry are broadening their reach by entering emerging markets, forging strategic alliances with local distributors, and customizing their product offerings to cater to different regions' unique aesthetic and therapeutic needs. This strategy enables companies to address the specific demands of diverse healthcare and cosmetic markets, ensuring improved product accessibility and availability. Aligning their solutions with regional preferences and regulatory requirements, these companies enhance their global presence and accelerate the adoption of advanced polynucleotide-based treatments across various healthcare and aesthetic environments.
Application Insights
The eye segment held the largest revenue share of 63.9% in 2024, driven by the growing demand for non-invasive solutions to address various eye-related concerns. Polynucleotide injectable improve the appearance of fine lines, wrinkles, and skin laxity around the delicate eye area, which is often a key concern for aging individuals. For instance, a study published in the Journal of Cosmetic Dermatology found that polynucleotide injections significantly improved periorbital skin quality, including increased elasticity and reduced appearance of crow's feet, without the need for more invasive procedures.
The forehead segment is anticipated to register the fastest CAGR over the forecast period. The market is driven by the rising consumer preference for minimally invasive aesthetic treatments that can address the visible signs of aging and enhance overall facial aesthetics. For instance, a recent study published in the Journal of Cosmetic Dermatology found that patients who received polynucleotide injections in the forehead area experienced a significant improvement in skin texture, reduced appearance of wrinkles, and enhanced facial rejuvenation without the need for more invasive procedures, including neurotoxins or dermal fillers.
End-use Insights
The MedSpas segment held the largest revenue share of 45.5% in 2024, driven by consumers' growing demand for non-invasive aesthetic treatments. Polynucleotides' unique properties, which enable them to stimulate collagen production, enhance skin texture, and diminish the appearance of fine lines and wrinkles, have made these injectable treatments a highly sought-after option among MedSpa clients seeking natural and long-lasting aesthetic improvements. For instance, an American Med Spa Association survey found 10,488 medical spas operating in the U.S. in 2023 as consumers increasingly sought these facilities to receive treatments such as polynucleotide injections.
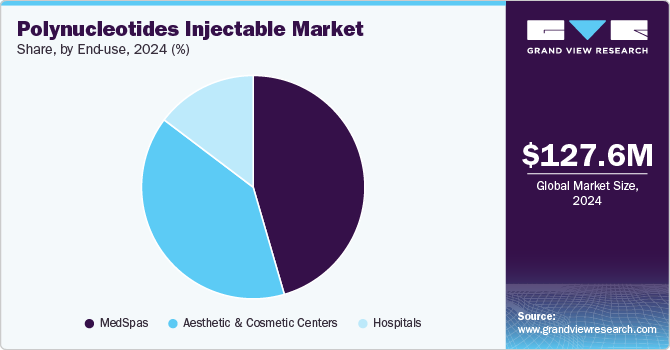
The Aesthetic & Cosmetic Centers segment is expected to grow at a significant rate over the forecast period. This rapid growth is driven by the rising consumer demand for minimally invasive aesthetic treatments that deliver natural-looking, long-lasting results. Aesthetic & Cosmetic Centers offer a wide range of services, including polynucleotide injectables, which are gaining popularity for improving skin texture, elasticity, and overall appearance. For instance, Pulse Light Clinic stated that polynucleotide injections deeply hydrate the skin, improving elasticity and youthful appearance. They stimulate natural collagen production for long-lasting skin health.
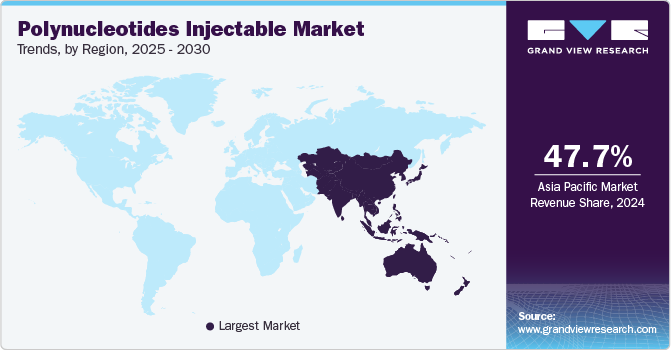
Regional Insights
The polynucleotides injectable market in North America has been experiencing significant growth in recent years due to increasing demand for advanced therapies and the presence of essential players in the region. The market is primarily driven by advancements in gene therapy and RNA-based therapeutics, as well as the launch of innovative products. Critically, polynucleotide-based injectables have not received approval from the FDA, thereby, it is expected that the market growth and share of the region will remain at the lower end during the forecast period.
U.S. Polynucleotides Injectable Market Trends
The U.S. polynucleotide injectable market is highly dynamic. It is projected to grow substantially due to the increasing adoption of personalized medicines and the growing demand for targeted therapies. The U.S. has a well-established biopharmaceutical industry, with several companies investing heavily in research and development activities related to polynucleotide-based therapies. The increasing geriatric population, which is more susceptible to skin aging and skin conditions, is also a significant factor driving the demand for effective aesthetic solutions. However, low confidence in the product from doctors & practitioners is expected to hinder growth.
According to a leading dermatologist in the U.S., “I don't know when the FDA is going to ever approve milt-derived chemical compounds for human use in Americans. I would not consider using polynucleotides until I see more long-term safety and outcome data.”
Europe Polynucleotides Injectable Market Trends
The European polynucleotide injectable market is also experiencing significant growth due to an increasing patient population base, easier regulatory approvals, and diverse needs from medical tourists. For instance, more than 140 injectables are approved in Europe as opposed to 22 in the U.S. Furthermore, novel technology penetration for aesthetic intervention is critically higher in the country, which drives growth. Some of the key brands available in the region are Nucleofill, Pluryal Silk, Rejuran, and others.
The UK polynucleotide injectable market has grown over the forecast period. The increasing demand for rejuvenation solutions and easier regulatory approvals drive the market's growth. For instance, polynucleotide injectable treatments are typically administered in two to three sessions spaced 2-3 weeks apart. Moreover, Dr. Mohan emphasizes that three treatments over this timeframe provide optimal results, highlighting the scientific foundation of polynucleotides. With a proven safety profile and over 1.8 million successful applications in both medical and aesthetic fields, these treatments are gaining trust among practitioners and patients.
Asia Pacific Polynucleotides Injectable Market Trends
The Asia Pacific polynucleotide injectable market held the largest revenue share of 47.7% in 2024 as the region is witnessing several key trends, including the emergence of domestic players offering low-cost products to expand market access and affordability. There is a strong presence of domestic competitors and multinational corporations in the region, with domestic players gaining success by catering to country-specific preferences. Innovations and improvements to treatments and products entering the Asia Pacific market also support sales growth, such as the recent approval of novel polynucleotide injectable products.
China's polynucleotide injectable market is growing over the forecast period, driven by rising consumer interest in advanced aesthetic treatments and skin rejuvenation solutions. Growing minimally invasive procedures, increasing disposable income, and a growing middle-class population. In addition, the entry of innovative products and advancements in injectable technology enhance treatment efficacy and patient satisfaction. With a strong focus on aesthetics and beauty trends, China is emerging as a key player in the polynucleotides injectable industry.
The Korean polynucleotide injectable market is anticipated to grow over the forecast period and is one of the key markets for polynucleotide injectables. Growing demand for rejuvenation solutions and increased demand for polynucleotide injections by Korean dermatologists drive the market's growth. According to the Injectable Skin Boosters article published in November 2024, among 235 board-certified Korean dermatologists specializing in cosmetic treatments, 88% reported using polynucleotide (PN) injections in their practices. In a separate clinical trial, Korean women who received four intradermal PN injections spaced two weeks apart experienced notable improvements in skin texture, including reduced pore size, enhanced skin thickness and tone, and diminished wrinkles and sagging, all without any significant adverse effects.
Latin American Polynucleotide Injectable Market Trends
The Latin American polynucleotide injectable market is experiencing significant growth due to a rising patient population base, easier regulatory approvals, and strategic initiatives by key players. For instance, in March 2024, Croma-Pharma partnered with Megalabs to expand access to its high-performance aesthetic products across Latin America and the Caribbean. The agreement was finalized in March 2024 and includes the divestiture of Croma's Brazil affiliate. It also grants Megalabs distribution rights for hyaluronic acid fillers, polynucleotide injectables, skincare products, and future technologies.
Brazil polynucleotide injectable market is anticipated to grow over the forecast period, driven by rising demand for non-invasive aesthetic solutions and skin rejuvenation treatments. The country’s beauty and wellness industry continues expanding, and more consumers seek personalized cosmetic procedures. Brazil's well-established market for cosmetic dermatology and its growing middle class create a productive environment for adopting advanced treatments like polynucleotide injectables. This trend is driven by the growing preference for precise and efficient treatments that cater to a broad range of consumers.
MEA Polynucleotide Injectable Market Trends
The MEA polynucleotide injectable market held a significant market share in 2024. Countries such as the UAE and Saudi Arabia, with their high disposable incomes and strong interest in advanced aesthetic procedures, are leading the market. In addition, increasing awareness of the benefits of polynucleotide-based therapies contributes to its rising popularity across the region. The expanding healthcare and aesthetic sectors in the MEA are enabling the growth of this innovative treatment.
Saudi Arabia polynucleotide injectable market is witnessing significant growth, driven by an increasing demand for non-invasive cosmetic treatments. With a rising focus on enhancing skin quality and addressing signs of aging, more individuals opt for polynucleotide injectables due to their effectiveness in rejuvenating and improving skin health. The growing popularity of aesthetic procedures in the country, along with an increasing number of healthcare providers offering these advanced treatments, is contributing to the market’s growth.
Key Polynucleotides Injectable Company Insights
Companies employ various strategies to enhance their market share, such as new product launches and partnerships. The growing demand for minimally invasive aesthetic treatments, the rising prevalence of skin conditions, and the increasing focus on personal well-being are the key drivers propelling the growth of the polynucleotide injectable industry.
Key Polynucleotides Injectable Companies:
The following are the leading companies in the polynucleotides injectable market. These companies collectively hold the largest market share and dictate industry trends.
- PHARMA RESEARCH
- BIOPLUS CO., LTD.
- LG Chem
- AMEELA
- Mastelli
- Pluryal
- Promoitalia Laboratories.
- Fox Pharma
- BR Pharm
- DermaFocus
Recent Developments
-
In May 2024, PharmaResearch revealed Rejuran's technological innovations in the Vietnamese market. Through this product launch, the company aims to expand its presence in the Vietnamese aesthetic and strengthen Rejuran's position as a global provider of innovative aesthetic solutions.
-
In October 2023, LG Chem and BR Pharm signed an MOU to collaborate on developing and approving HP Vitaran, a skin booster comprising polynucleotide (PN) derived from salmon and other fish reproductive cells, for the Chinese market. This partnership strives to leverage LG Chem's expertise and BR Pharm's regenerative medicine technology.
Polynucleotides Injectable Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 145.4 million
Revenue forecast in 2030
USD 293.4 million
Growth rate
CAGR of 15.1% from 2025 to 2030
Historical data
2018 - 2023
Forecast period
2025 - 2030
Quantitative units
Revenue in USD million and CAGR from 2025 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Application, end-use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Sweden; Denmark; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Argentina; Saudi Arabia; South Africa; UAE; Kuwait
Key companies profiled
PHARMA RESEARCH; BIOPLUS CO. LTD.; LG Chem; AMEELA; Mastelli; Pluryal; Promoitalia Laboratories; Fox Pharma; BR Pharm; DermaFocus
Customization scope
Free report customization (equivalent to up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Polynucleotides Injectable Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global polynucleotides injectable market report based on application, end-use, and region:
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Eyes
-
Lips
-
Forehead
-
Jawline & Cheekbones
-
Others
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
MedSpas
-
Aesthetic & Cosmetic Centers
-
Hospitals
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global polynucleotides injectable market size was estimated at USD 127.6 million in 2024 and is expected to reach USD 145.4 million in 2025.
b. The global polynucleotides injectables market is expected to grow at a compound annual growth rate of 15.1% from 2025 to 2030 to reach USD 293.4 milion by 2030.
b. The eye segment held the largest revenue share of 63.9% in the polynucleotide injectable market, driven by the growing demand for non-invasive solutions to address various eye-related concerns.
b. Some of the key players in polynucleotides injectables market are PHARMA RESEARCH, BIOPLUS CO., LTD., LG Chem, AMEELA, Mastelli, Pluryal, Promoitalia Laboratories, Fox Pharma, BR Pharm, and DermaFocus.
b. Technological advancements in biotechnology primarily drive the polynucleotides injectables market, as do the growing demand for skin rejuvenation, personalized medicine, and investments in research and development (R&D).
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










