- Home
- »
- Pharmaceuticals
- »
-
Pneumonia Therapeutics Market Size, Industry Report, 2030GVR Report cover
![Pneumonia Therapeutics Market Size, Share & Trends Report]()
Pneumonia Therapeutics Market (2024 - 2030) Size, Share & Trends Analysis Report By Product (Drugs, Vaccine, Oxygen Therapy), By Infection, By Route Of Administration, By End-use, By Region And Segment Forecasts
- Report ID: GVR-1-68038-492-5
- Number of Report Pages: 100
- Format: PDF
- Historical Range: 2018 - 2022
- Forecast Period: 2024 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Pneumonia Therapeutics Market Trends
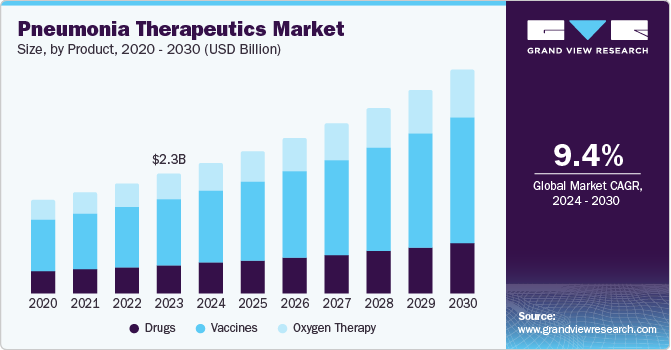
The global pneumonia therapeutics market size was valued at USD 2.28 billion in 2023 and is projected to grow at a CAGR of 9.4% from 2024 to 2030. The growing incidence of pneumococcal disease is a high impact rendering driver. Over the years, there has been a significant upsurge in the number of community-acquired and ventilator-associated bacterial pneumonia. Innovations in diagnostic technologies, in the realms of molecular diagnostics and point-of-care testing, have significantly transformed the landscape of pneumonia diagnosis and treatment. Molecular diagnostics, including techniques like polymerase chain reaction (PCR) and next-generation sequencing (NGS), enable the rapid and precise identification of pneumonia-causing pathogens at a genetic level. This precision allows for the detection of specific bacterial, viral, or fungal agents, facilitating targeted therapy and reducing the misuse of antibiotics. Point-of-care testing provides immediate diagnostic results at the site of patient care, such as in clinics or even at home, without the need for complex laboratory infrastructure.
The increasing prevalence of pneumonia, especially among age groups such as the elderly, children, and immunocompromised individuals, stands as a significant driver for the pneumonia therapeutics market. These populations are more susceptible to infections due to weakened immune systems and other health conditions. Contributing factors like air pollution exacerbate respiratory issues, making individuals more prone to contracting pneumonia. Smoking further damages lung function and reduces the body's ability to fight respiratory infections. Chronic diseases such as diabetes and asthma compromise immune defenses and lung health, increasing the risk of pneumonia. Additionally, crowded living conditions and inadequate access to healthcare in some areas can facilitate the spread of infectious agents that cause pneumonia. According to WHO report, Pneumonia accounts for 14% deaths of children under 5 years of age, it is the single largest infectious cause of death in children. This high disease burden creates a significant market demand for effective therapies.
Product Insights
The vaccine segment dominated the market and accounted for a share of 54.1% in 2023. Increasing awareness about the importance of preventive healthcare has significantly boosted the acceptance and demand for pneumonia vaccines. Public health campaigns and educational initiatives, spearheaded by governments, healthcare organizations, and non-profits, have played a crucial role in this shift. These initiatives have highlighted the severe risks and complications associated with pneumonia, especially among high-risk groups such as young children, the elderly, and those with compromised immune systems. By disseminating information through various media channels, community outreach programs, and healthcare provider endorsements, these campaigns have effectively communicated the benefits of vaccination in preventing pneumonia, driving the growth of segment.
Oxygen therapy is anticipated to grow at a moderate CAGR of 9.0% over the forecast period. This is attributed to the rising number of randomized controlled trials that have determined the effectiveness of oxygen therapy in intensive care units. In addition, the high clinical urgency to adopt systems for efficient management, vital in reducing complications, drives the demand for oxygen therapy to counter pneumococcal disease.
Infection Insights
Hospital-acquired pneumonia (HAP) infection accounted for the largest market revenue share in 2023. Advances in diagnostic tools and technologies have significantly improved the early detection and accurate diagnosis of hospital-acquired pneumonia (HAP), critical for effective patient management and outcomes. Innovations such as molecular diagnostics, including polymerase chain reaction (PCR) and next-generation sequencing (NGS), enable the rapid and precise identification of pathogens responsible for HAP, including multidrug-resistant strains. These advanced diagnostic methods can quickly differentiate between bacterial, viral, and fungal infections, allowing healthcare providers to tailor treatments to the specific causative agents. Point-of-care testing enhances this capability by providing immediate results at the bedside, facilitating swift clinical decisions, and promptly initiating appropriate therapy.
Community-acquired Pneumonia (CAP) is expected to grow at the fastest CAGR over the forecast period. The need for outpatient treatments, including oral antibiotics, has driven the market growth for therapeutics. The increasing awareness about early diagnosis has led to higher demand for therapeutics.
Route Of Administration Outlook
The parental segment held the largest revenue market share in 2023. Parenteral administration can ensure high bioavailability and effective delivery of medications. By administering therapeutics directly into the bloodstream via intravenous (IV) or intramuscular (IM) routes, parenteral methods bypass the gastrointestinal tract and potential issues with oral absorption, which can be problematic in critically ill patients. This direct delivery approach allows for the immediate and precise distribution of antibiotics and other drugs, which is crucial for managing acute and severe infections requiring rapid and potent intervention.
The oral segment is expected to grow significantly over the forecast period. Oral medications are generally preferred by patients due to their ease of use and convenience compared to intravenous or intramuscular administration. Oral drugs can be taken at home, which is more comfortable and less invasive, leading to higher patient compliance and adherence to treatment regimens.
End-use Outlook
The hospitals segment held the largest revenue market share in 2023. Hospitals are the primary segment for treating severe pneumonia cases who require mechanical ventilations. The high prevalence of hospitals is due to hospital - acquired-pneumonia (HAP)and ventilator -associated-pneumonia (VAP). Hospitals generally have the great infrastructure and experts to administer the complex antibiotic regimens. They also have access to advanced diagnostic tools for accurate and rapid diagnosis.
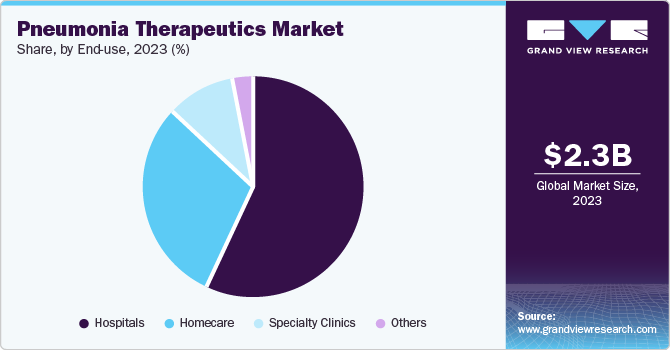
The homecare sector is projected to grow significantly over the forecast period. This is due to cost -reduction and prevention from hospitals infection. Oral antibiotics and inhaled medicines can easily be administered at home and the aging global population who are more susceptible from infection often prefers homecare for the treatment. Increasing healthcare prices are prompting insurers and healthcare systems to sell cost-powerful homecare solutions where appropriate. Additionally, government projects in many countries promote homecare as a way to reduce healthcare machine burden, driving the growth of segment.
Regional Insights
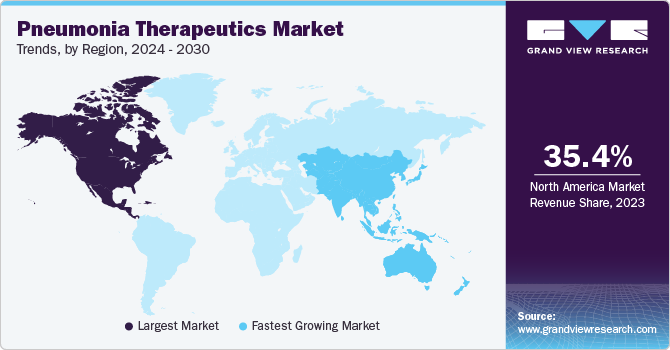
North America pneumonia therapeutics market held the highest revenue market share of 35.4% in 2023. Ongoing technological advancements in diagnostics and treatment options are a major driver of growth in the North American pneumonia therapeutics market. The region's status as a hub for pharmaceutical research and development has led to significant innovations in the field, including the development of novel antibiotics, antiviral drugs, and vaccines. These advancements have enhanced the accuracy of diagnostics, allowing for the rapid and precise identification of pneumonia pathogens. New antibiotics and antiviral drugs are increasingly effective against a broader range of pathogens, including multidrug-resistant strains, while the development of more targeted vaccines provides better prevention and protection against pneumonia.
U.S. Pneumonia Therapeutics Market Trends
The U.S. pneumonia therapeutics market dominated the North America market with highest revenue share in 2023. Increased healthcare spending in the U.S. plays a pivotal role in supporting the availability and adoption of advanced pneumonia treatments by bolstering investments in critical areas. Enhanced funding for healthcare infrastructure has led to the development of state-of-the-art medical facilities equipped with the latest diagnostic tools and treatment technologies. This investment in infrastructure ensures that healthcare providers have access to the resources needed for effective pneumonia management.
Europe Pneumonia Therapeutics Market Trends
Europe pneumonia therapeutics market held significant market revenue share in 2023. Increased awareness among healthcare providers and the public about pneumonia, its risk factors, and the benefits of early treatment and vaccination significantly drives the demand for effective therapeutics. Educational campaigns and health promotion initiatives play a crucial role in this process by disseminating vital information through various channels, including media, community outreach, and healthcare settings. These efforts aim to educate healthcare professionals and the public about the signs and symptoms of pneumonia, the importance of timely intervention, and the availability of preventive measures such as vaccines.
The UK pneumonia therapeutics market is expected to grow significantly over the forecast period. In the UK, a strong emphasis on preventive healthcare significantly drives the demand for pneumonia therapeutics. This focus is evident through widespread vaccination programs aimed at protecting high-risk groups such as the elderly, young children, and individuals with chronic conditions from pneumonia. The government and health organizations actively promote these vaccination campaigns as a critical strategy to reduce pneumonia incidence and its associated complications.
Asia Pacific Pneumonia Therapeutics Market Trends
Asia Pacific pneumonia therapeutics market is expected to grow at the fastest CAGR of 10.2% in forecast period. Adopting innovative healthcare delivery models, such as telemedicine and community-based health programs, significantly enhances access to pneumonia care by overcoming geographical and logistical barriers. Telemedicine enables patients in remote or underserved areas to consult with healthcare professionals via digital platforms, facilitating timely diagnosis and treatment without needing physical travel. This model is precious in regions with limited healthcare infrastructure, or areas affected by travel restrictions. Additionally, community-based health programs bring healthcare services closer to the population, focusing on preventative care, early detection, and management of pneumonia through localized clinics and outreach initiatives.
Japan pneumonia therapeutics market is expected to grow significantly over forecast period. Significant investment in pharmaceutical research and development in Japan plays a crucial role in fostering the advancement of novel pneumonia treatments. This investment supports a dynamic ecosystem where research activities, academic collaborations, and industry partnerships drive innovation. Leading Japanese pharmaceutical companies and educational institutions engage in cutting-edge research to discover and develop new antibiotics, antiviral drugs, and vaccines tailored to combat pneumonia. Clinical trials are a vital component of this process, providing the necessary evidence to validate the efficacy and safety of new treatments.
Key Pneumonia Therapeutics Company Insights
The competitive environment is marked by key players extensively deploying sustainability strategies AstraZeneca, Sanofi, Novartis are some of the most notable companies. These companies are involved in new product development initiatives, mergers & acquisitions, and geographical expansion.
-
AstraZeneca is a global biopharmaceutical company known for its commitment to innovative healthcare solutions, including pneumonia therapeutics. The company offers a range of products designed to address various aspects of pneumonia treatment and prevention. Key offerings include antibiotics such as Zoladex (a broad-spectrum antibiotic used to treat bacterial infections, including pneumonia) and Faslodex (used in specific cases related to cancer with secondary infections).
-
Eli Lilly and Company is a global pharmaceutical company. The company's product offerings in the pneumonia therapeutics market focus on innovative therapies to improve patient outcomes. Notable among their products is Cyramza (ramucirumab), which, although primarily used for oncology, can be relevant in managing pneumonia complications in cancer patients.
Key Pneumonia Therapeutics Companies:
The following are the leading companies in the pneumonia therapeutics market. These companies collectively hold the largest market share and dictate industry trends.
- AstraZeneca
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- Mylan N.V.
- Teva Pharmaceutical Industries Ltd.
- Sanofi
- Novartis AG
- Sun Pharmaceutical Industries Ltd.
- Aurobindo Pharma
- Lupin
Recent Development
-
In June 2024, Merck received approval from the U.S. Food and Drug Administration (FDA) for its latest pneumococcal vaccine designed for adult use. This newly approved vaccine is a significant addition to Merck's portfolio, aimed at providing enhanced protection against pneumococcal diseases, including invasive infections and pneumonia, which pose serious health risks to adults, particularly those with underlying health conditions.
-
In June 2024, the U.S. Food and Drug Administration (FDA) granted approval for CAPVAXIVE (Pneumococcal 21-valent Conjugate Vaccine. CAPVAXIVE is expected to play a crucial role in reducing the incidence of these potentially life-threatening conditions, particularly among older adults and individuals with compromised immune systems.
-
In December 2023, Wockhardt completed the Phase 3 clinical trial of Nafithromycin (WCK 4873), an antibiotic intended for the treatment of pneumonia. This important study evaluated the efficacy and safety of Nafithromycin in combating bacterial pneumonia, marking a significant milestone in the development of this advanced therapeutic. The completion of this trial positions Wockhardt to advance toward regulatory submissions, potentially offering a new treatment option in the fight against pneumonia, particularly in the face of increasing antibiotic resistance.
Pneumonia Therapeutics Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 2.48 billion
Revenue forecast in 2030
USD 4.26 billion
Growth rate
CAGR of 9.4% from 2024 to 2030
Base year for estimation
2023
Historical data
2018 - 2022
Forecast period
2024 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, infection, route of administration, end-use, region
Regional scope
North America, Europe, Asia Pacific, Latin America, MEA
Country scope
U.S., Canada, Mexico, UK, Germany, Spain, France, Italy Japan, China, India, Australia, South Korea, Thailand, Brazil, Argentina, South Africa, Saudi Arbia, UAE, Kuwait
Key companies profiled
AstraZeneca; Eli Lilly and Company; F. Hoffmann-La Roche Ltd.; Mylan N.V.; Teva Pharmaceutical Industries Ltd.; Sanofi; Novartis AG Sun Pharmaceutical Industries Ltd.; Aurobindo Pharma; Lupin
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Pneumonia Therapeutics Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global pneumonia therapeutics market report based on product, infection, route of administration, end-use, and region.
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Drugs
-
Vaccine
-
Oxygen Therapy
-
-
Infection Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospital-acquired Pneumonia (HAP)
-
Community-acquired Pneumonia (CAP)
-
Ventilator-associated Pneumonia (VAP)
-
-
Route of Administration Outlook (Revenue, USD Million, 2018 - 2030)
-
Oral
-
Parenteral
-
Others
-
-
End Use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Homecare
-
Specialty Clinics
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
Saudi Arabia
-
UAE
-
South Africa
-
Kuwait
-
-
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










