
Personalized Medicine Biomarkers Market Size, Share & Trends Analysis Report By Application (Early Detection, Treatment Selection, Monitoring), By Indication (Oncology, Neurology), By Region, And Segment Forecasts, 2025 - 2030
- Report ID: GVR-4-68039-996-3
- Number of Report Pages: 120
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2024
- Forecast Period: 2025 - 2030
- Industry: Healthcare
Market Size & Trends
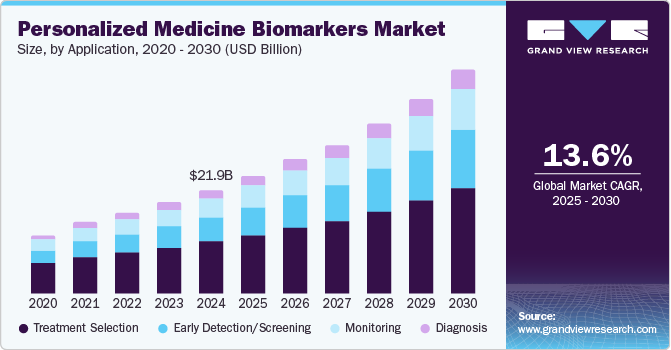
The global personalized medicine biomarkers market size was estimated at USD 21.88 billion in 2024 and is anticipated to grow at a CAGR of 13.6% from 2025 to 2030. The market is witnessing growth due to factors such as increasing demand for personalized treatment regime, increasing prevalence of chronic diseases among the population, and technological advancements in the diagnosis and detection of chronic diseases. The market growth is further propelled by the additional benefits offered by biomarkers, which include the ability to detect health conditions in their early stages, improve patient survival, and reduce treatment costs.
The market expansion is fueled by the rising prevalence of chronic diseases, as well as the growing need for precision medicine in oncology, cardiology, and other therapeutic areas, which is reshaping the landscape of healthcare. As per the February 2024 report released by the CDC, roughly 129 million individuals in the U.S. are impacted by at least one significant chronic illness. These conditions necessitate more tailored treatment approaches, driving demand for biomarkers that can predict patient responses to therapies. According to the National Cancer Institute at the National Institutes of Health, it is projected that in 2024, approximately 9,620 children aged 0 to 14 will be diagnosed with cancer, and an estimated 1,040 will succumb to the disease. As diagnostic tools improve, the demand for biomarkers that can accurately detect and monitor cancer in children has surged. The growing emphasis on precision medicine, which seeks to customize healthcare based on individual characteristics, is further propelling this market.
In addition, technological advancements play a pivotal role in the growth of the personalized biomarkers market. Innovations in next-generation sequencing (NGS), bioinformatics, and molecular imaging have revolutionized biomarker discovery and validation processes. These technologies enable the identification of specific biomarkers associated with various cancers, allowing for more accurate and early diagnosis. NGS, for instance, facilitates the comprehensive analysis of cancer genomes, leading to the discovery of novel genetic mutations that can serve as potential biomarkers. These technological innovations not only enhance the precision of diagnostics but also contribute to the development of targeted therapies, further driving market growth.
Moreover, research in this field has intensified, with a focus on identifying novel biomarkers that can not only detect cancer at an early stage but also predict treatment response and disease progression. In March 2022, the National Cancer Institute (NCI) introduced the Molecular Characterization Initiative, a program offering tumor molecular characterization, commonly referred to as biomarker testing, to children and adolescents. The initiative primarily targets individuals newly diagnosed with tumors who are undergoing treatment at hospitals. This research is crucial for developing personalized treatment strategies, which are particularly important in oncology, where minimizing long-term side effects is a priority. Advancements in technologies such as next-generation sequencing (NGS) and liquid biopsies have also accelerated biomarker discovery, enabling more comprehensive and non-invasive testing options.
Regulatory support for personalized medicine is another critical factor driving market growth. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), are actively promoting the development of companion diagnostics that are essential for the safe and effective use of targeted therapies. This support is fostering an environment conducive to innovation and encouraging pharmaceutical companies to invest in biomarker research. Furthermore, increasing awareness among healthcare professionals and patients about the benefits of personalized medicine is boosting the adoption of biomarker-based tests.
Furthermore, the growing emphasis on patient-centric healthcare models is shaping the personalized medicine biomarkers market. As healthcare systems move towards more individualized approaches, there is an increased focus on understanding patients' unique biological profiles. This trend is leading to the development of more precise diagnostics and therapeutics that can enhance treatment efficacy and minimize adverse effects. By tailoring medical isnterventions to individual patients, healthcare providers can improve overall patient satisfaction and outcomes, further propelling the growth of the market.
Application Insights
The treatment selection segment dominated the market with the largest market share of 50.2% in 2024. Based on the molecular characteristics of the tumor, personalized medicine biomarkers help choose the most suitable patients for early-phase clinical trials. They also provide information regarding action mechanism of a particular medicine. The focus of oncology drug development programs has shifted from the creation of non-specific cytotoxic chemotherapies to the creation of molecularly targeted therapeutics as a result of the identification of particular mutations that efficiently perform the prediction for a given drug in a molecularly defined patient group. Moreover, with an enhanced selection of treatment the probability to tackle the condition increases which further propels the demand for personalized medicine biomarkers.
Early detection/screening is expected to grow at a significant CAGR of 15.8% over the forecast period. The growth of the segment can be attributed to the need to provide early treatment. For patients who display the biomarker linked to a therapy's response, the probability of survival is dependent on the course of treatment. For instance, the expression of the HER2/neu protein is a prognostic biomarker of breast cancer. Moreover, KRAS is a frequently mutated oncogene in colorectal cancer and a biomarker for anti-EGFR monoclonal antibody therapy resistance. Moreover, the development of novel technologies to help in the early detection of various conditions is further propelling the growth of the market.
Indication Insights
Oncology dominated the market with the largest share of 35.0% in 2024, driven by the increasing prevalence of cancer worldwide. According to the World Health Organization, cancer cases are projected to rise to 29.5 million by 2040, necessitating more precise diagnostic and therapeutic approaches. Personalized medicine, which tailors treatment based on individual genetic and molecular profiles, enhances the efficacy of cancer therapies and minimizes adverse effects. According to GLOBOCAN, there were 18,741,966 cancer cases globally in 2022. Among these, 9,566,825 cases were reported in men, while 9,175,141 cases were recorded in women. The demand for biomarkers that can predict patient responses to targeted therapies and immunotherapies is rapidly increasing, facilitating earlier detection and improving overall patient outcomes in oncology, thus propelling market growth in this segment.
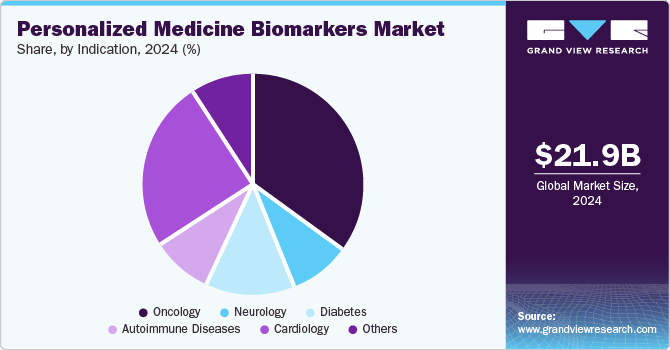
The diabetes segment is the fastest-growing segment and is expected to grow at a CAGR of 17.4% over the forecast period. The diabetes segment is experiencing notable growth within the personalized medicine biomarkers market, driven by the rising global prevalence of diabetes. According to the IDF Diabetes Atlas, the global number of individuals living with diabetes is on the rise and is expected to increase by 46% from 2020 to 2045, while the world’s population is anticipated to grow by just 20%. Personalized medicine, which focuses on tailoring treatments based on individual biomarkers, plays a crucial role in diabetes management by identifying patients at risk for complications and optimizing therapeutic strategies. The demand for biomarkers that can predict glycemic control, insulin sensitivity, and other metabolic responses is increasing, fostering advancements in targeted therapies and improving patient outcomes in diabetes care.
Regional Insights
The North America personalized medicine biomarkers market dominated the global market and accounted for 51.93% of revenue share in 2024, which can be attributed to increasing funding for biomarkers aided by technological advancements, high disposable income, and the presence of key market players operating in the region. The U.S. held the largest share of personalized medicines in the region owing to increasing private and public initiatives and raising awareness among the population about chronic diseases and treatments. Moreover, key players are focusing on developing novel products to maintain their dominance over the market. For instance, in February 2022, FoundationOne CDx was approved by the FDA approval as a companion diagnostic for Keytruda to categorize patients with microsatellite instability-high solid tumors.
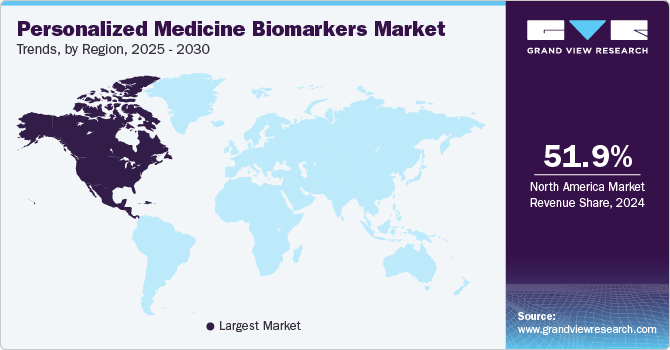
U.S. Personalized Medicine Biomarkers Market Trends
The U.S.personalized medicine biomarkers market held a significant share of North America market in 2024, driven by advancements in genomics and increased demand for targeted therapies. Rising investments in research, regulatory support for companion diagnostics, and a growing emphasis on precision medicine in oncology and chronic disease management are further propelling market expansion in this sector.
Europe Personalized Medicine Biomarkers Market Trends
The Europe personalized medicine biomarkers market driven by advancements in personalized medicine and intensive research efforts in oncology. Innovations such as next-generation sequencing and proteomics are improving biomarker discovery and diagnostic accuracy, enabling more tailored treatment approaches. European research institutions are increasingly focused on identifying novel biomarkers specific to cancers and other chronic disorders, fostering innovation in personalized medicine. According to the European Society of Cardiology, approximately 45% of individuals over the age of 65 are affected by cardiovascular diseases (CVDs), highlighting the significant health challenges faced by this demographic. In 2022, nearly 4 million people in Europe died from CVDs. This combination of technological advancements and dedicated research initiatives is significantly propelling the growth of the personalized medicine biomarkers market across the region, enhancing the potential for targeted therapies and improved patient outcomes.
The UK personalized medicine biomarkers market is expected to show significant growth driven by increasing research and development investments and enhanced funding for innovative projects. This financial support is facilitating the discovery of novel biomarkers and advancing precision medicine initiatives, leading to improved diagnostics and targeted therapies, ultimately enhancing patient outcomes across the region.
The Germany personalized medicine biomarkers market is experiencing significant growth, largely due to the rising prevalence of chronic disorders such as cancer, diabetes, and cardiovascular diseases. This increasing burden on healthcare systems is driving demand for targeted diagnostics and tailored therapies, fostering advancements in personalized medicine and enhancing patient care in the region.
Asia Pacific Personalized Medicine Biomarkers Market Trends
The Asia Pacific personalized medicine biomarkers market is expected to grow at the fastest CAGR over the forecast period. The growth of the market in the region is owing to growing healthcare reforms in the region aided by improved healthcare infrastructure, a growing population, and an increase in the number of companies entering the market. The Asia Pacific region has a large population and high cancer prevalence. Thus, the usage of screening tests for cancer has increased in the past few years owing to growing government initiatives, such as free screening for breast cancer and increased collaborations for the distribution and supply of these tests.
The China personalized medicine biomarkers market is growing at a lucrative rate. Presence of government funding and initiatives in the region further fuels regional growth. For instance, The Chinese Personal Medicine Initiative which is expected to be funded with USD 9.0 billion by 2030, aims to provide better healthcare provisions mainly for oncology.
Latin America Personalized Medicine Biomarkers Market Trends
The Latin America personalized medicine biomarkers market exhibits high growth potential for the personalized medicine biomarkers market. Driven by improvements in healthcare infrastructure and strong government support. Many countries in the region are investing in modernizing their healthcare systems, which enhances access to advanced diagnostic tools and personalized treatment options. Government initiatives aimed at promoting research and development in personalized medicine are further accelerating market expansion. Additionally, as awareness of the benefits of tailored therapies increases among healthcare providers and patients, demand for biomarkers that facilitate precision medicine is on the rise. These factors collectively contribute to the positive trajectory of the personalized medicine biomarkers market in Latin America.
Middle East and Africa Personalized Medicine Biomarkers Market Trends
The MEA personalized medicine biomarkers market growth driven by the increasing prevalence of chronic disorders, such as diabetes, cardiovascular diseases, and cancer. As healthcare systems in the region focus on improving diagnosis and treatment through personalized medicine, the demand for innovative biomarkers is rising, enhancing patient outcomes.
The Saudi Arabia personalized medicine biomarkers market growth is driving the market, largely supported by government funding aimed at enhancing healthcare innovation. Increased investment in research initiatives and the establishment of advanced healthcare facilities are fostering the development of novel biomarkers, which are essential for improving personalized treatment strategies and patient care in the region.
Key Personalized Medicine Biomarkers Company Insights
The market is highly competitive due to several strategic initiatives such as new product launches, mergers and acquisitions, and regional expansion, undertaken by key market players to increase their global footprints. Companies are diversifying their product portfolios to address a broader range of applications.
Key Personalized Medicine Biomarkers Companies:
The following are the leading companies in the personalized medicine biomarkers market. These companies collectively hold the largest market share and dictate industry trends.
- Laboratory Corporation of America Holding
- Quest Diagnostics Incorporated
- Agilent Technologies, Inc.
- Genome Medical, Inc.
- Coriell Life Sciences.
- Thermo Fisher Scientific Inc.
- NeoGenomics Laboratories
- FOUNDATION MEDICINE, INC.
- Illumina, Inc.
- Guardant Health
View a comprehensive list of companies in the Personalized Medicine Biomarkers Market
Recent Developments
-
In August 2024, Paige announced the launch of OmniScreen, an innovative AI-driven biomarker module designed to analyze more than 505 genes and identify 1,228 molecular biomarkers from standard H&E-stained digital pathology slides.
-
In August 2024, Illumina, Inc. announced that its in vitro diagnostic TruSight Oncology Comprehensive test has received Food and Drug Administration approval, along with its first two companion diagnostic indications. This comprehensive test analyzes over 500 genes to profile a patient’s solid tumor, enhancing the chances of identifying immuno-oncology biomarkers or clinically actionable biomarkers that facilitate targeted therapy options or clinical trial participation.
-
In June 2024, Sapience Therapeutics, Inc., a biotechnology company focused on peptide therapeutics for oncogenic and immune dysregulation, presented new clinical and biomarker data from its Phase 2 ST101 study.
Personalized Medicine Biomarkers Market Report Scope
|
Report Attribute |
Details |
|
The market size value in 2025 |
USD 24.88 billion |
|
The revenue forecast in 2030 |
USD 47.16 billion |
|
Growth rate |
CAGR of 13.6% from 2025 to 2030 |
|
Actual data |
2018 - 2024 |
|
Forecast period |
2025 - 2030 |
|
Quantitative units |
Revenue in USD billion/million, and CAGR from 2025 to 2030 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Application, indication, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
|
Country scope |
U.S.; Canada; Mexico; Germany; France; UK; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait |
|
Key companies profiled |
Laboratory Corporation of America Holding; Quest Diagnostics Incorporated; Agilent Technologies, Inc.; Genome Medical, Inc.; Coriell Life Sciences; Thermo Fisher Scientific Inc.; NeoGenomics Laboratories; FOUNDATION MEDICINE, INC.; Illumina, Inc.; Guardant Health. |
|
Customization scope |
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. Explore purchase options |
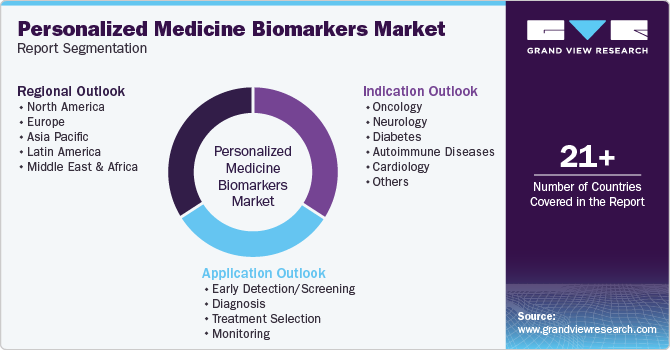
Global Personalized Medicine Biomarkers Market Report Segmentation
This report forecasts revenue growth and provides an analysis on the latest trends in each of the sub-segments from 2018 to 2030. For the purpose of this report, Grand View Research has segmented the global personalized medicine biomarkers market report on the basis of application, indication, and region.
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Early Detection/Screening
-
Diagnosis
-
Treatment Selection
-
Monitoring
-
-
Indication Outlook (Revenue, USD Million, 2018 - 2030)
-
Oncology
-
By Type
-
Breast Cancer
-
Lung Cancer
-
Colon Cancer
-
Others
-
-
By Circulating Biomarkers
-
Circulating Tumor Cells(CTCs)
-
Circulating Cell-free DNA(cfDNA)
-
Extracellular Vesicles (EVs)
-
Other Circulating Biomarkers
-
-
-
Neurology
-
Diabetes
-
Autoimmune Diseases
-
Cardiology
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global personalized medicine biomarkers market size was estimated at USD 21.88 billion in 2024 and is expected to reach USD 24.88 billion in 2025.
b. The global personalized medicine biomarkers market is expected to grow at a compound annual growth rate of 13.6% from 2025 to 2030 to reach USD 47.16 billion by 2030.
b. North America dominated the personalized medicine biomarkers market with a share of 51.93% in 2024. This is attributable to increasing funding for biomarkers aided with technological advancements, high disposable income and the presence of key market players operating in the region
b. Some key players operating in the personalized medicine biomarkers market include Laboratory Corporation of America Holding, Quest Diagnostics Incorporated, Agilent Technologies, Inc., Genome Medical, Inc., Coriell Life Sciences., Thermo Fisher Scientific Inc., NeoGenomics Laboratories, FOUNDATION MEDICINE, INC., Illumina, Inc., Guardant Health.
b. Key factors that are driving the market growth include factors such as increasing demand for personalized medicine, increasing prevalence of chronic diseases among the population, and technological advancements in field of diagnosis and detection of fatal diseases.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."




