- Home
- »
- Clinical Diagnostics
- »
-
Pediatric Allergy Diagnostics Market Size, Share Report 2030GVR Report cover
![Pediatric Allergy Diagnostics Market Size, Share & Trends Report]()
Pediatric Allergy Diagnostics Market (2024 - 2030) Size, Share & Trends Analysis Report By Product (Instruments, Consumables), By Allergen (Food, Inhaled, Drug), Test (In Vivo Test, In Vitro Test), By Region, And Segment Forecasts
- Report ID: GVR-4-68040-442-0
- Number of Report Pages: 120
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2024 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Pediatric Allergy Diagnostics Market Trends
The global pediatric allergy diagnostics market size was estimated at USD 2.44 billion in 2023 and is projected to grow at a CAGR of 10.1% from 2024 to 2030. Environmental factors such as increased pollution, changing lifestyles, and urbanization have contributed to a higher incidence of allergies among children. Food allergies, allergic rhinitis, and asthma are particularly common, with many children requiring ongoing treatment and monitoring. According to the CDC, in 2021, 27.2% of children were diagnosed with at least one of the three main allergic conditions: seasonal allergies, eczema, or food allergies. Seasonal allergies were the most common, affecting 18.9% of children, followed by eczema at 10.8% and food allergies at 5.8%. This increasing prevalence has increased the demand for diagnostic tests and effective therapeutic options. As awareness of pediatric allergies continues to rise among healthcare providers and parents, the market is expected to expand, providing better management and treatment solutions for affected children.
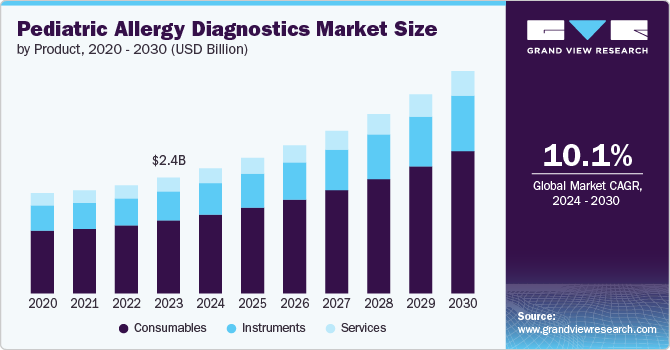
In addition, technological advancements in allergy diagnostics are significantly influencing the market. Innovations such as next-generation sequencing, component-resolved diagnostics (CRD), and microarray-based tests have enhanced the accuracy and speed of detection. These advanced diagnostic tools allow more precise identification of specific allergens, enabling tailored treatment plans for pediatric patients. Early and accurate diagnosis is crucial in managing allergies effectively, reducing the risk of severe allergic reactions, and improving the quality of life for children. The adoption of these cutting-edge technologies is expected to drive market growth as healthcare providers increasingly rely on advanced diagnostics to deliver personalized care. Moreover, integrating digital health tools and telemedicine in allergy diagnostics further supports market expansion by making diagnostics more accessible.
Moreover, Public health campaigns, educational programs, and initiatives by healthcare organizations have heightened awareness about the importance of early diagnosis and treatment of allergies in children. For instance, in July 2024, the Indian Academy of Pediatrics (IAP) launched the "Tackling Cough With Care" campaign to raise awareness about pediatric cough management. Parents are now more informed about the signs and symptoms of allergies, leading to earlier consultation with healthcare providers and timely intervention. This increased awareness has also increased the demand for allergy testing and therapeutic options. Furthermore, healthcare professionals are better equipped to diagnose and manage pediatric allergies, thanks to ongoing training and access to updated guidelines. As awareness spreads, the market will likely see sustained growth driven by proactive management of allergic conditions in children.
Market Concentration & Characteristics
The pediatric allergy diagnostics market is marked by a high degree of innovation, driven by technological advancements and a better understanding of allergic conditions. New diagnostic tools, such as component-resolved diagnostics (CRD) and next-generation sequencing, allow for more precise identification of specific allergens, enabling personalized treatment plans for children. Moreover, innovations like non-invasive testing methods and rapid diagnostic kits make allergy testing more accessible and less stressful for pediatric patients, significantly enhancing the market's growth and potential.
Regulations significantly impact the market by ensuring diagnostic tests' safety, accuracy, and efficacy. Stringent guidelines from regulatory bodies, such as the FDA and EMA, govern the approval and use of diagnostic tools, influencing market dynamics. While these regulations help maintain high standards of care, they can also slow the introduction of new technologies and increase development costs. Compliance with these regulations is crucial for market players to ensure the reliability and acceptance of their diagnostic solutions.
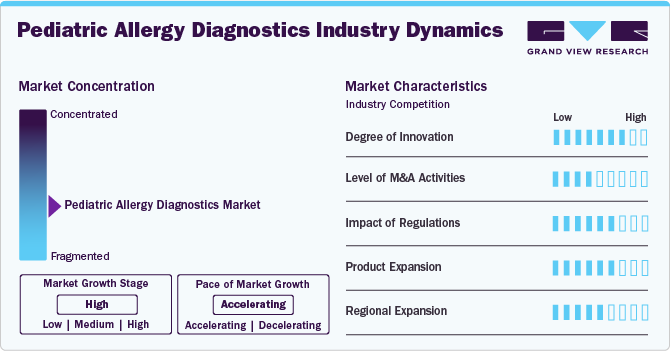
The market has seen moderate mergers and acquisitions (M&A) activity as companies seek to expand their product portfolios and enhance their technological capabilities. For instance, in August 2020, Nestlé Health Science and Aimmune announced a partnership to establish a global leader in food allergy prevention and treatment. Aimmune's recently approved therapy, Palforzia, will enhance Nestlé Health Science's portfolio as the first and only FDA-approved treatment designed to reduce the frequency and severity of peanut allergies in children. Larger pharmaceutical and diagnostics firms are acquiring smaller, innovative companies to gain access to advanced diagnostic tools and new technologies, such as molecular allergy diagnostics. These M&A activities are driven by the need to stay competitive in a growing market and to meet the increasing demand for accurate and efficient allergy diagnostics in pediatric care.
Product expansion in the market is robust, driven by the development of innovative diagnostic tools and therapies. Companies are introducing advanced technologies such as component-resolved diagnostics, multiplex assays, and non-invasive testing methods to enhance accuracy and ease of use. In addition, there is a growing focus on creating age-appropriate and user-friendly products tailored to children. This expansion aims to address a wider range of allergic conditions and improve early detection and management of allergies in pediatric patients.
Regional expansion in pediatric allergy diagnostics is accelerating as companies aim to enter emerging and underserved markets. Efforts are focused on increasing access to advanced diagnostic tools in Asia-Pacific, Latin America, and the Middle East. Local partnerships, regulatory approvals, and tailored solutions are being pursued to address specific regional needs and challenges. This expansion is driven by growing awareness of pediatric allergies and increasing demand for effective diagnostic solutions worldwide.
Product Insights
The consumables segment held the largest share of 62.95% in 2023 and is expected to grow at the fastest CAGR of 10.33% over the forecast period, driven by increasing demand for allergy testing and management solutions. Key players like Thermo Fisher Scientific, Siemens Healthineers, and Abbott are leading the development of innovative diagnostic consumables, including test kits, reagents, and sample collection tools. The rising prevalence of pediatric allergies and advancements in diagnostic technologies fuel this demand. Innovations such as multiplex assays and improved allergy panels are enhancing the accuracy and efficiency of tests. These advancements meet growing market needs and support the expansion of diagnostic capabilities in pediatric care.
The instruments market in pediatric allergy diagnostics is experiencing notable growth, driven by technological advancements and increasing demand for accurate and efficient testing. These instruments facilitate high-throughput testing and precise allergen identification, which is essential for managing pediatric allergies effectively. The market growth is fueled by rising allergy prevalence among children and the need for reliable, rapid diagnostics. Innovations in instrumentation, such as integration with digital platforms and enhanced sensitivity, are further propelling market expansion by improving diagnostic accuracy and patient outcomes.
Allergen Insights
The inhaled segments held the largest share of 45.40% in 2023due to increasing awareness of respiratory allergies and advancements in inhaled diagnostic technologies. Products like inhaled allergen challenge tests are becoming more prevalent, helping to accurately diagnose conditions such as asthma and allergic rhinitis in children. This growth is supported by rising air pollution levels, which contribute to higher rates of respiratory allergies. According to a June 2024 report from the American Lung Association, asthma is more prevalent among male children (7.0%) compared to female children (5.4%). As urbanization and pollution increase globally, there is a growing need for effective diagnostic tools to assess and manage allergy-induced respiratory issues, driving market expansion in this segment.
The food segment is expected to grow at a significant CAGR of 11.77% over the forecast period due to the rising incidence of food allergies among children. According to 2022 statistics from the CDC, food allergies are a significant public health concern, affecting approximately 8% of children in the U.S. This equates to 1 in 13 children, or roughly 2 students per classroom. The increasing prevalence of food allergies drives demand for specialized diagnostic tools and tests to accurately identify and manage these conditions. As awareness grows and more children are diagnosed, the market for food allergy diagnostics is expected to continue its upward trajectory.
Test Insights
The in vitro test segment held the largest share of 51.76% in 2023 and is expected to grow at the fastest CAGR over the forecast period, driven by advances in diagnostic technology and increased demand for precise allergy testing. In vitro tests, such as component-resolved diagnostics (CRD) and specific IgE assays, offer high accuracy and efficiency in identifying allergens. The growing prevalence of allergies among children and the need for reliable, non-invasive diagnostic methods fuel this market expansion. Innovations in in vitro testing, including enhanced sensitivity and multiplex capabilities, further support the segment's growth by providing comprehensive and detailed allergen profiles essential for effective allergy management.
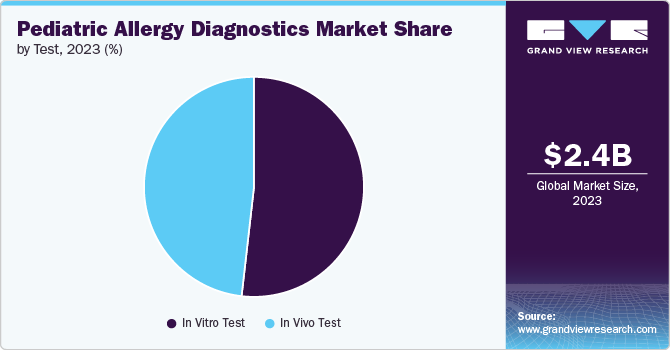
The in vivo test segment in the market is experiencing steady growth due to its critical role in diagnosing and managing allergies. Skin prick and intradermal tests are widely used to identify allergic reactions to specific allergens. This segment benefits from the rising incidence of allergies in children and an increasing demand for accurate diagnostic methods. The market is also supported by advancements in test methodologies and the development of safer, more efficient testing protocols. As awareness of pediatric allergies grows, the demand for reliable in vivo testing drives market expansion, offering essential insights for tailored treatment plans.
Regional Insights
North America pediatric allergy diagnostics market dominated the overall global market and accounted for the 36.44% of revenue share in 2023, driven by the rising incidence of allergies and supportive government initiatives. Factors such as increased awareness, early diagnosis, and advancements in diagnostic technologies contribute to this growth. According to 2023 data from the Asthma and Allergy Foundation of America, the annual cost of nasal allergies ranges between USD 3.0 and USD 4.0 billion, highlighting the economic burden and the urgent need for effective diagnostics. Favorable government policies and healthcare infrastructure investments further support the region's market expansion.
U.S. Pediatric Allergy Diagnostics Market Trends
Thepediatric allergy diagnostics marketin the U.S. held a significant share of the North American market in 2023, fueled by the increasing prevalence of allergies among children. According to a 2023 CDC report, 18.9% of U.S. children suffer from seasonal allergies, and 5.8% have food allergies. Common allergens include peanuts, milk, eggs, fish, shellfish, soy, tree nuts, and wheat. The rising incidence of these allergies drives demand for advanced diagnostic tools that can accurately identify and manage allergic reactions. As awareness grows and more children are diagnosed, the U.S. market for pediatric allergy diagnostics is expected to continue expanding, addressing a critical healthcare need.
Europe Pediatric Allergy Diagnostics Market Trends
The pediatric allergy diagnostics market in Europe is experiencing significant growth driven by rising demand for effective diagnostic solutions amid the increasing prevalence of allergies. According to AllergyUK, over 150 million Europeans suffer from chronic allergic diseases, with projections indicating that by 2025, half of the EU population could be affected. In the UK alone, 40% of children have been diagnosed with an allergy. This surge in allergy cases is fueling the need for advanced diagnostic tools tailored for pediatric patients, driving market expansion across Europe as healthcare systems seek to manage and mitigate the growing allergy burden.
The UK pediatric allergy diagnostics market is experiencing significant growthdue to healthcare improvements and technological advancements. Enhanced diagnostic technologies, such as more accurate and non-invasive tests, drive better allergy management. These innovations, coupled with a focus on improving healthcare infrastructure, are significantly boosting the market's expansion in the UK.
The pediatric allergy diagnostics market in Germany is experiencing significant growth, driven by increasing awareness and demand for accurate diagnostic tools. According to BfR, in 2021, approximately 4% of children and adults in Germany had food allergies, with many also experiencing respiratory and gastrointestinal allergic reactions. This prevalence underscores the need for advanced diagnostics, fueling market expansion in the country.
Asia Pacific Pediatric Allergy Diagnostics Market Trends
The pediatric allergy diagnostics market in Asia Pacificis experiencing the fastest growth, driven by significant healthcare infrastructure and technological advancements. Countries in the region are investing in modern diagnostic facilities, enhancing access to cutting-edge allergy testing. Technological innovations, such as more precise and less invasive diagnostic methods, further propel market expansion. The study called “The burden of allergic diseases in the Indian Subcontinent: Barriers and Challenges,” published in 2020, stated that the phase 3 international study of asthma and allergy in children (ISASC) reported that an overall prevalence of wheezing was 7% in children aged between 6 & 7 in India and about 10% to 20% prevalence was seen in children aged 13-14 years. As awareness of pediatric allergies increases, the demand for advanced diagnostics is rising, contributing to the rapid market growth in Asia.
China pediatric allergy diagnostics market is growing, driven by the increasing prevalence of allergies among children and rising demand for accurate diagnostics. As urbanization and environmental changes contribute to higher allergy rates, more parents seek reliable diagnostic solutions. This surge in allergy cases is fueling demand for advanced diagnostic tools, leading to significant market expansion in China as healthcare providers strive to meet this growing need.
The pediatric allergy diagnostics market in Japan is experiencing growth driven by the increasing prevalence of food allergies among young children. A 2020 study by NIH reported that caregiver-reported immediate food allergy prevalence was 7.6% at age 1, 6.7% at age 2, and 4.9% at age 3. Hen egg allergy was most common (5.3% at age 1), followed by cow milk (2.1%) and wheat (0.5%). This rising allergy trend is boosting demand for advanced diagnostic solutions in Japan.
Latin America Pediatric Allergy Diagnostics Market Trends
The pediatric allergy diagnostics market in Latin Americais experiencing significant growthdue to increasing allergy prevalence and enhanced awareness programs. Government support and initiatives are boosting market development. According to a 2020 survey-based study, the prevalence of anaphylaxis among Mexican schoolchildren was 1.2%. This rising awareness and governmental backing drive demand for advanced allergy diagnostic solutions across the region.
Middle East and Africa Pediatric Allergy Diagnostics Market Trends
The pediatric allergy diagnostics market in MEAis expanding and is driven by increasing awareness and rising allergy rates. A 2019 study, “Prevalence of Food Allergy Among Schoolchildren in Kuwait,” found that 4.1% of schoolchildren aged 11-14 had food allergies. Major allergens included fish (1.6%), eggs (2.7%), peanuts (1.3%), shellfish (1.3%), and tree nuts (1.2%), highlighting the need for improved diagnostic solutions in the region.
Saudi Arabia pediatric allergy diagnostics market is expanding due to increased healthcare investment and improved infrastructure. The government is boosting funding for advanced diagnostic technologies and healthcare facilities, enhancing the availability of allergy testing. These investments drive market growth by addressing the rising prevalence of allergies in children.
Key Pediatric Allergy Diagnostics Company Insights
The market is highly competitive, with key players such as Biopharm AG, Omega Diagnostics Group PLC, and AESKU.GROUP GmbH holds significant positions. The major companies are undertaking various strategies, such as regional expansion, acquisitions, mergers, collaborations, and new product development, to serve their customers' unmet needs.
Key Pediatric Allergy Diagnostics Companies:
The following are the leading companies in the pediatric allergy diagnostics market. These companies collectively hold the largest market share and dictate industry trends.
- R-Biopharm AG
- EUROIMMUN Medizinische Labordiagnostika AG (PerkinElmer, Inc.)
- AESKU.GROUP GmbH
- bioMérieux
- Thermo Fisher Scientific, Inc.
- Stallergenes Greer
- Minaris Medical America, Inc.
- Siemens Healthcare GmbH
- Omega Diagnostics Group PLC
- HYCOR Biomedical
- Lincoln Diagnostics, Inc.
Recent Developments
-
In July 2024, EUROIMMUN Medizinische Labordiagnostika AG introduced chemiluminescence immunoassays (ChLIA), designed to swiftly and accurately identify sensitivities to allergens. These assays enable precise measurement of IgE antibodies in the blood, which are responsible for triggering allergic reactions.
-
In December 2023, Thermo Fisher Scientific entered into a long-term, exclusive distribution agreement with AESKU.GROUP in the U.S. This partnership allows Thermo Fisher to offer a wide range of autoimmune diagnostics and automated instrumentation, enhancing their portfolio with AESKU’s advanced IFA testing technologies.
-
In May 2023, Metropolis Healthcare Limited, a diagnostic service provider, introduced a new testing platform utilizing Component Resolved Diagnostics (CRD). This advanced platform aims to enhance allergy diagnosis for the diverse population in India, offering more precise identification of various allergy types.
Pediatric Allergy Diagnostics Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 2.63 billion
Revenue forecast in 2030
USD 4.68 billion
Growth rate
CAGR of 10.07% from 2024 to 2030
Actual data
2018 - 2023
Forecast period
2024 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, allergen, test, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Norway; Denmark; Sweden; China; Japan; India; Australia; Thailand; South Korea; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Biopharm AG; EUROIMMUN Medizinische Labordiagnostika AG (PerkinElmer, Inc.); AESKU.GROUP GmbH; bioMérieux; Thermo Fisher Scientific, Inc.; Stallergenes Greer; Minaris Medical America, Inc.; Siemens Healthcare GmbH; Omega Diagnostics Group PLC; HYCOR Biomedical; Lincoln Diagnostics, Inc.
Customization scope
Free report customization (equivalent up to 8 analyst working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
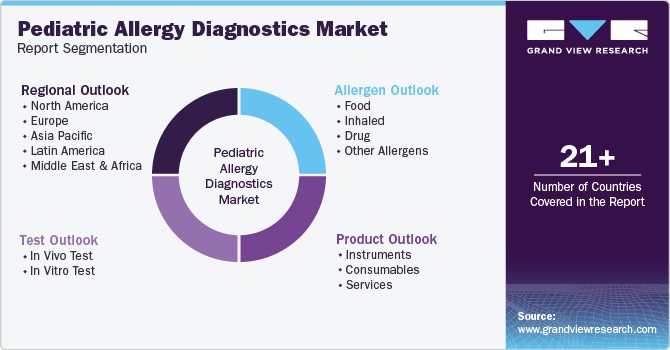
Global Pediatric Allergy Diagnostics Market Report Segmentation
This report forecasts revenue growth at country levels and provides an analysis on the latest industry trends and opportunities in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global pediatric allergy diagnostics market report based on product, allergen, test, and region:
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Instruments
-
Consumables
-
Services
-
-
Allergen Outlook (Revenue, USD Million, 2018 - 2030)
-
Food
-
Dairy Products
-
Poultry Product
-
Tree Nuts
-
Peanuts
-
Shellfish
-
Wheat
-
Soys
-
Other Food Allergens
-
-
Inhaled
-
Drug
-
Other Allergens
-
-
Test Outlook (Revenue, USD Million, 2018 - 2030)
-
In vivo Test
-
Skin Prick Test
-
Intradermal Test
-
Patch Test
-
-
In vitro Test
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Norway
-
Denmark
-
Sweden
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
Mexico
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global pediatric allergy diagnostics market size was estimated at USD 2.44 billion in 2023 and is expected to reach USD 2.63 billion in 2024.
b. The global pediatric allergy diagnostics market is expected to grow at a compound annual growth rate of 10.07% from 2024 to 2030 to reach USD 4.68 billion by 2030.
b. North America dominated the pediatric allergy diagnostics market with a share of 36.44% in 2023. This is attributable to rising incidence of allergies and supportive government initiatives. Factors such as increased awareness, early diagnosis, and advancements in diagnostic technologies contribute to this growth.
b. Some key players operating in the pediatric allergy diagnostics market include Biopharm AG; EUROIMMUN Medizinische Labordiagnostika AG (PerkinElmer, Inc.); AESKU.GROUP GmbH; bioMérieux; Thermo Fisher Scientific, Inc.; Stallergenes Greer; Minaris Medical America, Inc.; Siemens Healthcare GmbH; Omega Diagnostics Group PLC; HYCOR Biomedical; Lincoln Diagnostics, Inc.
b. Key factors that are driving the market growth include environmental factors such as increased pollution, changing lifestyles, and urbanization have contributed to a higher incidence of allergies among children. Food allergies, allergic rhinitis, and asthma are particularly common, with many children requiring ongoing treatment and monitoring.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










