
Paroxysmal Nocturnal Hemoglobinuria Treatment Market Size, Share & Trends Analysis Report By Treatment (Medication, Stem Cell Transplant, Blood Transfusion), By Region, And Segment Forecasts, 2025 - 2030
- Report ID: GVR-2-68038-622-6
- Number of Report Pages: 100
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
PNH Treatment Market Size & Trends
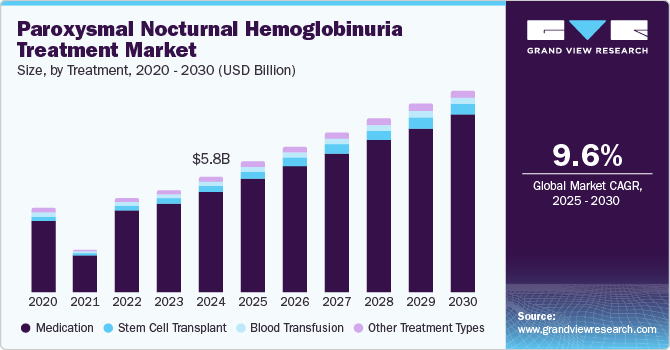
The global paroxysmal nocturnal hemoglobinuria treatment market size was estimated at USD 5.75 billion in 2024 and is projected to grow at a CAGR of 9.6% from 2025 to 2030. The increasing prevalence of paroxysmal nocturnal hemoglobinuria (PNH) is driven by better awareness and improved diagnostic techniques. More cases are identified as more people realize the disease, leading to a growing demand for treatment. Although PNH is a rare disorder, advancements in diagnostics are helping doctors detect it more easily, leading to more diagnoses. The recognition of PNH as a condition is expanding, helping to increase its visibility and improve patient outcomes. With these advancements, the number of diagnosed patients is expected to rise, further boosting the market for the PNH treatment industry.
Advances in treatment options for PNH have significantly improved with the development of targeted therapies, particularly complement inhibitors such as eculizumab and ravulizumab. These medications effectively manage hemolysis, the destruction of red blood cells, and mitigate associated complications. Continuous innovation in this field leads to next-generation therapies offering enhanced safety profiles and more convenient administration methods. Designed to ensure long-term effectiveness, these new treatments positively impact patient outcomes. As these therapeutic options evolve, they drive substantial growth in the PNH treatment industry.
Improved diagnostic techniques, such as flow cytometry, have made it easier to detect PNH with greater accuracy. This allows for earlier diagnosis and timely treatment, which is essential for better patient outcomes. In addition, advancements in genetic testing and biomarkers are helping doctors identify patients who may respond well to specific treatments. These improved diagnostic methods ensure patients receive the most appropriate care at the right time. Hence, the ability to diagnose and treat PNH has significantly improved, contributing to better disease management in the PNH treatment industry.
Treatment Insights
The medication segment dominated the market with a revenue share of 85.3% in 2024, driven by the increasing prevalence of PNH and the growing awareness of available treatments. The introduction of novel therapies targeting the underlying mechanisms of PNH has led to improved patient outcomes, driving demand for these medications. In addition, the effectiveness of these treatments in managing symptoms and reducing complications associated with PNH has contributed to their widespread adoption among healthcare providers. The rise in healthcare spending and investments in research and development further support the growth of this segment as pharmaceutical companies strive to innovate and enhance treatment options for patients suffering from this rare blood disorder.
The stem cell transplant segment is projected to grow significantly over the forecast period, which can be attributed to advancements in transplant techniques and increased success rates. As more healthcare facilities adopt stem cell transplantation as a viable treatment option for PNH, patient outcomes have improved significantly. This is boosted by ongoing research into the role of stem cells in regenerating blood cells and correcting the genetic defects associated with PNH. Furthermore, the growing body of clinical evidence supporting the long-term benefits of stem cell transplants has led to increased acceptance among clinicians and patients alike, facilitating a shift toward this innovative treatment modality.
Regional Insights
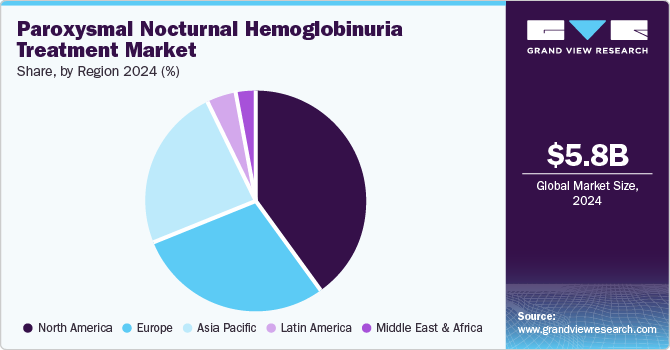
North America paroxysmal nocturnal hemoglobinuria treatment market held the highest revenue share of 40.4% in 2024, driven by a combination of factors including a high prevalence of neurological disorders and chronic pain conditions. The region benefits from advanced healthcare infrastructure, which supports the adoption of cutting-edge neurostimulation technologies. In addition, leading medical device companies significant investments in research and development have resulted in innovative products that enhance treatment efficacy. The increasing acceptance of neurostimulation therapies among healthcare providers and patients contributes to the growth of the PNH treatment industry, alongside favorable reimbursement policies that facilitate access to these advanced treatments.
U.S. Paroxysmal Nocturnal Hemoglobinuria Treatment Market Trends
The U.S. paroxysmal nocturnal hemoglobinuria treatment market dominated North America, with a significant revenue share in 2024, driven by the availability of specialized therapies that cater specifically to PNH patients. The presence of key pharmaceutical companies focusing on developing targeted treatments has significantly enhanced patient access to effective therapies. Moreover, increasing awareness among healthcare professionals about PNH and its management has led to timely diagnosis and treatment initiation, further boosting the growth of the PNH treatment industry. The supportive clinical environment and robust healthcare system in the U.S. also play crucial roles in facilitating advancements in PNH treatment options.
Europe Paroxysmal Nocturnal Hemoglobinuria Treatment Market Trends
Europe paroxysmal nocturnal hemoglobinuria treatment market held a substantial market share in 2024, fueled by an increasing number of clinical trials aimed at evaluating new therapies for PNH. The region's regulatory framework encourages innovation while ensuring patient safety, leading to a steady influx of novel treatments entering the market. In addition, a growing collaboration between academic institutions and pharmaceutical companies has accelerated research efforts focused on PNH therapies. The rising prevalence of rare diseases such as PNH has also prompted greater investment from both the public and private sectors, enhancing treatment accessibility across European countries.
Asia Pacific Paroxysmal Nocturnal Hemoglobinuria Treatment Market Trends
Asia Pacific paroxysmal nocturnal hemoglobinuria treatment market is expected to register the highest CAGR of 10.3% over the forecast period, which can be attributed to increasing healthcare expenditures and improving access to advanced medical treatments. Rapid economic growth in countries such as China and India has led to enhanced healthcare infrastructure and rising awareness about rare diseases among healthcare professionals and patients alike. Moreover, the growing focus on personalized medicine is driving demand for targeted therapies that address specific patient needs within this region. As more stakeholders recognize the importance of treating rare diseases such as PNH, investment in research and development is likely to increase.
The China paroxysmal nocturnal hemoglobinuria treatment market dominated the Asia Pacific, with a significant revenue share in 2024 due to its large population base and rising incidence rates of blood disorders. The Chinese government’s initiatives to improve healthcare access have facilitated better diagnosis and treatment options for patients with PNH. Moreover, local pharmaceutical companies are increasingly investing in innovative therapies tailored to the Chinese population, enhancing treatment availability. The collaboration between domestic manufacturers and international firms fosters technological advancements that contribute to effective management strategies for PNH within the country.
Key Paroxysmal Nocturnal Hemoglobinuria Treatment Company Insights
Some key companies operating in the market are Alexion Pharmaceuticals, Inc.;Apellis Pharmaceuticals; Novartis AG; F. Hoffmann-La Roche Ltd, and Genentech, Inc. Companies are undertaking strategic initiatives, such as mergers, acquisitions, and product launches, to expand their market presence and address the evolving healthcare demands in the paroxysmal nocturnal hemoglobinuria treatment market.
-
Alexion Pharmaceuticals, Inc. provides a range of innovative treatments for PNH, including the first approved therapy, Soliris (eculizumab), which effectively reduces hemolysis. They also offer ULTOMIRIS (ravulizumab), a long-acting alternative for less frequent dosing. Recently, the company introduced Voydeya (danicopan), an oral Factor D inhibitor designed as an add-on therapy for patients experiencing extravascular hemolysis while on Soliris or ULTOMIRIS.
-
Apellis Pharmaceuticals offers innovative solutions for PNH treatment with its primary product, EMPAVELI (pegcetacoplan). This therapy is the first targeted complement C3 inhibitor approved for adults with PNH, effective for both treatment-naïve patients and those transitioning from C5 inhibitors such as Soliris and Ultomiris. The company also provides the ApellisAssist program, which offers support services such as insurance assistance and education for eligible patients.
Key Paroxysmal Nocturnal Hemoglobinuria Treatment Companies:
The following are the leading companies in the paroxysmal nocturnal hemoglobinuria treatment market. These companies collectively hold the largest market share and dictate industry trends.
- Alexion Pharmaceuticals, Inc.
- Apellis Pharmaceuticals
- Novartis AG
- F. Hoffmann-La Roche Ltd
- Genentech, Inc.
- BioCryst Pharmaceuticals, Inc.
- Regeneron Pharmaceuticals Inc.
- Sanofi
- Pfizer Inc.
- Amgen Inc.
Recent Developments
-
In October 2023, Apellis Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) approved the EMPAVELI Injector, a device designed to facilitate the self-administration of EMPAVELI (pegcetacoplan) for patients with PNH. This approval is intended to enhance patient convenience and adherence to treatment by allowing individuals to administer their medication at home.
-
In June 2021, Alexion Pharmaceuticals announced that the U.S. FDA approved ULTOMIRIS (ravulizumab-cwvz) for treating adults with PNH. This approval marked a significant advancement in the management of PNH, offering patients a long-acting complement inhibitor that allows for less frequent dosing compared to previous therapies. The company highlighted that ULTOMIRIS would provide an important treatment option for patients, improving their quality of life while effectively managing the disease.
Paroxysmal Nocturnal Hemoglobinuria Treatment Market Report Scope
|
Report Attribute |
Details |
|
Market size value in 2025 |
USD 6.45 billion |
|
Revenue forecast in 2030 |
USD 9.96 billion |
|
Growth rate |
CAGR of 9.6% from 2025 to 2030 |
|
Base year for estimation |
2024 |
|
Historical data |
2018 - 2023 |
|
Forecast period |
2025 - 2030 |
|
Quantitative units |
Revenue in USD billion, and CAGR from 2025 to 2030 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, trends |
|
Segments covered |
Treatment, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country scope |
U.S., Canada, Mexico, UK, Germany, France, Italy, Spain, Denmark, Sweden, Norway, Japan, China, India, Australia, South Korea, Thailand, Brazil, Argentina, South Africa, Saudi Arabia, UAE, Kuwait |
|
Key companies profiled |
Alexion Pharmaceuticals, Inc.; Apellis Pharmaceuticals; Novartis AG; F. Hoffmann-La Roche Ltd; Genentech, Inc.; BioCryst Pharmaceuticals, Inc.; Regeneron Pharmaceuticals Inc.; Sanofi; Pfizer Inc.; Amgen Inc.. |
|
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. Explore purchase options |
Global Paroxysmal Nocturnal Hemoglobinuria Treatment Market Report Segmentation
This report forecasts global, regional, and country revenue growth and analyzes the latest industry trends in each sub-segment from 2018 to 2030. For this study, Grand View Research has segmented the global paroxysmal nocturnal hemoglobinuria treatment market report based on treatment, and region:
-
Treatment Outlook (Revenue, USD Billion, 2018 - 2030)
-
Medication
-
Stem Cell Transplant
-
Blood Transfusion
-
Other Treatment Types
-
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global paroxysmal nocturnal hemoglobinuria (PNH) market size was estimated at USD 2.7 billion in 2019 and is expected to reach USD 3.0 billion in 2020.
b. The global paroxysmal nocturnal hemoglobinuria (PNH) market is expected to grow at a compound annual growth rate of 11.2% from 2017 to 2025 to reach USD 5.8 billion by 2025.
b. Medication segment dominated the paroxysmal nocturnal hemoglobinuria (PNH) market with a share of 77.8% in 2019. This is attributable to the introduction of novel PNH therapies and increasing R&D activities by major players.
b. Some key players operating in the paroxysmal nocturnal hemoglobinuria (PNH) market include Alexion Pharmaceuticals, Takeda Pharmaceutical Company Limited (Shire Plc), Akari Therapeutics, Apellis Pharmaceuticals, Ra Pharmaceuticals, Achillion Pharmaceuticals, Amgen Inc., Regeneron Pharmaceuticals, Inc., and F. Hoffmann-La Roche AG.
b. Key factors that are driving the market growth include introduction of novel PNH therapies, robust pipeline, and increasing R&D investments.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."




