
Non-alcoholic Steatohepatitis Clinical Trials Market Size, Share & Trends Analysis Report By Phase (Phase I, Phase II, Phase III, Phase IV), By Study Design, By Region, And Segment Forecasts, 2025 - 2030
- Report ID: GVR-4-68039-911-4
- Number of Report Pages: 120
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2024
- Forecast Period: 2025 - 2030
- Industry: Healthcare
Market Size & Trends
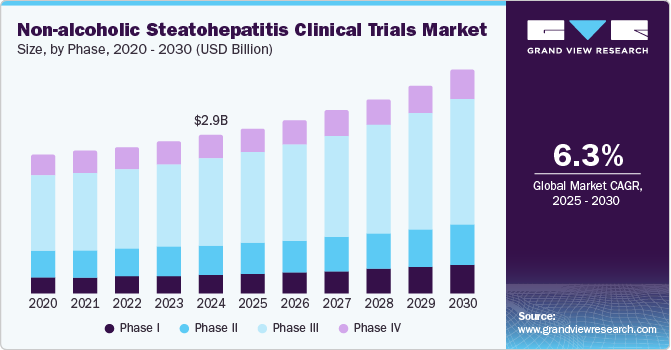
The global non-alcoholic steatohepatitis clinical trials market size was estimated at USD 2.92 billion in 2024 and is projected to grow at a CAGR of 6.26% from 2025 to 2030. The market growth is mainly due to increased drug R&D, rising prevalence of obesity & diabetes mainly due to sedentary lifestyles, and unmet medical needs. According to the data published by WHO in March 2024, approximately 2.5 billion adults aged 18 and older were overweight in 2022, including over 890 million living with obesity. This figure represented 43% of adults globally. Thus, increasing cases of obesity and metabolic diseases like type 2 diabetes would lead to the growing incidence of Non-alcoholic Fatty Liver Disease (NAFLD), which can progress to non-alcoholic steatohepatitis (NASH).
Furthermore, increasing research and development activities by market players to develop NASH therapeutics further contributes to the market growth. Since there is currently just one approved therapy for NASH, this lack of treatment options has spurred extensive research and investment into drug development. The market for NASH therapies is expected to grow as more pharmaceutical companies enter space, conducting clinical trials to develop innovative treatments. Companies such as Intercept Pharmaceuticals and Madrigal Pharmaceuticals are significantly investing in clinical trials for NASH, underscoring the demand for effective therapeutic solutions. As a result, clinical trials in NASH are receiving greater attention, with new drug candidates targeting various stages of the disease.
In addition, growing advancements in biomarkers, imaging technologies, and non-invasive diagnostic tools are also advancing NASH clinical trials. Emerging non-invasive methods, such as elastography and blood biomarkers, are making it easier to diagnose patients at an early stage of the disease. This helps better identify suitable candidates for clinical trials and accelerates recruitment. Thus, with improved diagnostic precision, researchers can also stratify patients more effectively, leading to better trial outcomes and more robust clinical data.
Phase Insights
The phase III segment dominated the market, accounting for 54.3% of the total revenue share in 2024. Phase III trials are essential for confirming the efficacy and safety of new therapies before they can be submitted for regulatory approval. These large-scale, pivotal studies typically involve a diverse patient population and provide the final evidence to support the commercialization of novel NASH treatments. Thus, the growing demand for effective NASH therapies has subsequently boosted the demand for Phase III trials,with numerous investigational drugs currently undergoing evaluation in these advanced stages.
Phase I is projected to witness the fastest growth during the forecast period owing to an increasing number of novel drug candidates entering early-stage clinical trials for Non-alcoholic Steatohepatitis (NASH). Phase I trials are crucial for assessing new therapies' safety, tolerability, and pharmacokinetics in healthy volunteers or patients. As more pharmaceutical companies focus on developing innovative treatments for NASH, the number of compounds advancing to Phase I studies is expected to increase in the coming years.
Study Design Insights
The interventional studies segment dominated the market in 2024 owing to an increasing focus on evaluating new therapeutic interventions. Moreover, growing research and development studies to test novel drug candidates, including both monotherapies and combination therapies for NASH. These studies aimed to assess the efficacy and safety of investigational treatments, given the growing prevalence of metabolic diseases like obesity and type 2 diabetes, which contribute to the rise in NASH cases. Thus, the aforementioned factors are further contributing to segment growth.
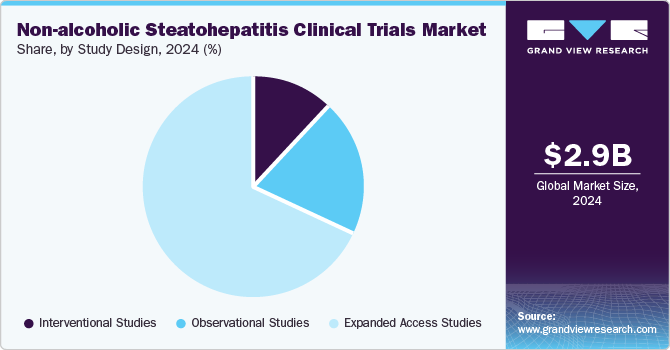
The observational studies segment is projected to witness the fastest growth in the coming years. These studies play a crucial role in understanding the natural progression of NASH, as well as identifying potential biomarkers and risk factors associated with the disease. By monitoring patients over extended periods without intervening with experimental treatments, observational studies provide valuable insights into disease outcomes, patient behavior, and the effectiveness of real-world treatments.
Regional Insights
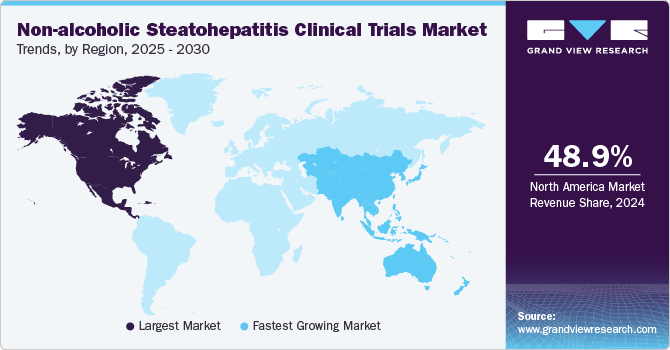
North America non-alcoholic steatohepatitis clinical trials market dominated and accounted for a 48.9% share in 2024. The growth in the region is attributed to the region’s advanced healthcare system, large patient population, and strong research infrastructure. The prevalence of metabolic diseases, including obesity, diabetes, and cardiovascular diseases, which are risk factors for NASH, has contributed to a high demand for NASH treatment research. Moreover, growing research and development activities by the market players towards the development of effective therapies has also accelerated the conduction of clinical trials in the region.
U.S. Non-alcoholic Steatohepatitis Clinical Trials Market Trends
The U.S. non-alcoholic steatohepatitis clinical trials market is projected to be driven due to country’s well-established healthcare infrastructure, advanced research facilities, coupled with growing prevalence of NASH-related conditions such as obesity and diabetes. In addition, increasing government support towards the rapid development of NASH therapies, with several clinical trials investigating novel drug candidates and treatments for liver fibrosis associated with NASH is also contributing the country’s market growth. For instance, March 2024, U.S. FDA approved Rezdiffra, the first treatment for NASH, a type of fatty liver disease.
Europe Non-alcoholic Steatohepatitis Clinical Trials Market Trends
The Europe non-alcoholic steatohepatitis clinical trials market is anticipated to witness lucrative growth over the projected period. The growth is due toadvanced clinical research capabilities coupled with significant support by government and various academic institutions to conduct NASH trials. Moreover, increasing collaboration between pharmaceutical companies, CROs, regulatory bodies, and healthcare providers further fuel market growth.
The UK non-alcoholic steatohepatitis clinical trials market is anticipated to experience considerable growth over the forecast period. The market's growth is mainly due to the stringent regulatory landscape, the presence of major pharmaceutical & biopharmaceutical companies, and the increasing adoption of innovative trial approaches. In addition, the strong infrastructure of the National Health Service (NHS), which provides a robust platform for conducting trials, is a key driver for market growth.The UK government has emphasized addressing liver diseases, including NASH, as part of its public health initiatives.
The non-alcoholic steatohepatitis clinical trials market in Germany is expected to grow at a considerable rate over the forecast period owing to the presence of strong pharmaceutical industry and research institutions. The country’s hospitals and academic centers conduct numerous studies related to NASH treatments, especially in areas like liver fibrosis progression, insulin resistance, and non-invasive diagnostic methods. Germany’s regulatory body, the Paul-Ehrlich-Institut, works closely with the European Medicines Agency (EMA), ensuring that NASH treatments meet high safety and efficacy standards.
Asia Pacific Non-alcoholic Steatohepatitis Clinical Trials Market
The non-alcoholic steatohepatitis clinical trials market in Asia Pacific is expected to grow at the highest CAGR over the forecast period.The growth can be attributed to the increasing prevalence of metabolic disorders, such as diabetes and obesity. Moreover, the rapidly growing patient pool and increasing healthcare investments by several public and private players make the region attractive for NASH trials. The country’s such as India offers significant opportunities for cost-effective clinical trials, despite some regulatory and infrastructure challenges, which is further contributing towards the market growth.
China non-alcoholic steatohepatitis clinical trials market is projected to witness significant growth in the coming years owing to both local and global treatments for NASH, with significant support from the Chinese government for healthcare research and development. In March 2024, Chipscreen Biosciences, based in Shenzhen, China, announced promising results from a Phase 2 trial of its metabolic dysfunction-associated steatohepatitis (MASH) drug candidate, chiglitazar. The new drug candidate reduces liver fat in Phase 2 trial.
The non-alcoholic steatohepatitis clinical trials market in Japan is expected to witness lucrative growth over the forecast period due to the increasing prevalence of metabolic syndrome and liver diseases. Japanese pharmaceutical companies, such as Eisai and Takeda, are actively involved in NASH drug development, with several trials focused on advanced liver fibrosis and cirrhosis.
India non-alcoholic steatohepatitis clinical trials market is poised to grow in the coming years owing to the increasing prevalence of Non-alcoholic Fatty Liver Disease (NAFLD). According to data published by IQVIA, approximately 5% of patients in India are diagnosed with Non-alcoholic Fatty Liver Disease (NAFLD) which may progress to Non-alcoholic Steatohepatitis (NASH). This more severe liver condition poses significant health risks. A study conducted in a rural area of West Bengal highlighted the increasing prevalence of NAFLD in India, revealing that 75% of individuals diagnosed with the condition did not have obesity. These factors signify that NAFLD may affect a broader, more diverse patient population, including those without typical risk factors. Thus, increasing the prevalence of NAFLD would further contribute to the country’s market growth.
MEA Non-alcoholic Steatohepatitis Clinical Trials Market Trends
The non-alcoholic steatohepatitis clinical trials market in MEA is projected to grow at a lucrative rate.The market's growth is mainly due to rising disease prevalence, an expanding drug pipeline, better diagnostic tools, and increasing investments in clinical research. However, challenges related to patient recruitment, regional healthcare disparities, and regulatory hurdles may restrict the region’s market growth.
Saudi Arabia non-alcoholic steatohepatitis clinical trials market is projected to witness considerable growth rate. Saudi Arabia has one of the highest rates of obesity and type 2 diabetes globally. According to the World Health Organization (WHO), more than 35% of adults in the country are obese, while around 25% are living with diabetes. These prevalent conditions significantly elevate the risk of developing Non-alcoholic Fatty Liver Disease (NAFLD), which can progress to Non-alcoholic Steatohepatitis (NASH). As a result, Saudi Arabia is becoming a key location for NASH-focused clinical trials, offering a large and relevant patient population for research studies.
Key Non-alcoholic Steatohepatitis Clinical Trials Company Insights
Key players operating in the market are Pfizer Inc., Novartis AG, Icon Plc, LabCorp, and Allergan Plcare undertaking various strategic initiatives, such as the signing of the new partnership agreement, collaborations, mergers & acquisitions, geographic expansion, aiming to strengthen their services, & manufacturing capacities, thus providing a competitive advantage. For instance, in December 2023, Pfizer Inc. announced the Phase 2b Results for Danuglipron, an Oral GLP-1R Agonist, in Adults with Obesity.
Key Non-alcoholic Steatohepatitis Clinical Trials Companies:
The following are the leading companies in the non-alcoholic steatohepatitis clinical trials market. These companies collectively hold the largest market share and dictate industry trends.
- Pfizer Inc.
- Novartis AG
- Icon Plc
- Laboratory Corporation of America
- AbbVie Inc.
- Cadila Healthcare Ltd.
- Shire Plc (Takeda Pharmaceuticals)
- Eli Lilly
- Novo Nordisk
- Gilead Sciences Inc.
- Glaxosmith Kline
- Arrowhead Pharmaceuticals
View a comprehensive list of companies in the Non-alcoholic Steatohepatitis Clinical Trials Market
Recent Developments
-
In March 2024, Madrigal Pharmaceuticals Receives FDA Approval for Rezdiffra (resmetirom) to Treat Patients with Noncirrhotic Nonalcoholic Steatohepatitis (NASH) and Moderate to Advanced Liver Fibrosis.
-
In June 2024, Boehringer Ingelheim announced results from a sub-analysis of its Survodutide Phase II trial, showing that up to 64.5% of adults with fibrosis stages F2 and F3 (moderate to advanced liver scarring) experienced improvement in fibrosis without worsening of metabolic dysfunction-associated steatohepatitis (MASH).
-
In June 2024, Eli Lilly and Company announced the detailed findings from the SYNERGY-NASH Phase 2 study, which involved 190 patients, with or without type 2 diabetes. The study aimed to assess the investigational use of tirzepatide in adults with biopsy-confirmed metabolic dysfunction-associated steatohepatitis (MASH) and stage 2 or 3 fibrosis.
Non-alcoholic Steatohepatitis Clinical Trials Market Report Scope
|
Report Attribute |
Details |
|
Market size value in 2025 |
USD 3.05 billion |
|
Revenue forecast in 2030 |
USD 4.14 billion |
|
Growth rate |
CAGR 6.26% from 2025 to 2030 |
|
Actual data |
2018 - 2024 |
|
Forecast period |
2025 - 2030 |
|
Quantitative units |
Revenue in USD million/billion and CAGR from 2025 to 2030 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Phase, study design, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
|
Country scope |
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; South Korea; Thailand; Australia; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait |
|
Key companies profiled |
Pfizer Inc.; Novartis AG; Icon Plc; Laboratory Corporation of America; AbbVie Inc.; Cadila Healthcare Ltd.; Shire Plc (Takeda Pharmaceuticals); Eli Lilly; Novo Nordisk; Gilead Sciences Inc.; Glaxosmith Kline; Arrowhead Pharmaceuticals |
|
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. Explore purchase options |
Global Non-alcoholic Steatohepatitis Clinical Trials Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global non-alcoholic steatohepatitis clinical trials market report based on phase, study design, and region:
-
Phase Outlook (Revenue, USD Million, 2018 - 2030)
-
Phase I
-
Phase II
-
Phase III
-
Phase IV
-
-
Study Design Outlook (Revenue, USD Million, 2018 - 2030)
-
Interventional Studies
-
Observational Studies
-
Expanded Access Studies
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Norway
-
Sweden
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
MEA
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global non-alcoholic steatohepatitis clinical trials market size was estimated at USD 2.92 billion in 2024 and is expected to reach USD 3.05 billion in 2025.
b. The global non-alcoholic steatohepatitis clinical trials market is expected to grow at a compound annual growth rate of 6.26% from 2025 to 2030 to reach USD 4.14 billion by 2030.
b. North America dominated the market for NASH clinical trials and accounted for the largest revenue share of 48.9% in 2024.
b. Some key players operating in the NASH clinical trials market include Pfizer Inc.; Novartis AG; Icon Plc; LabCorp; AbbVie Inc.; Cadila Healthcare Ltd.; Shire Plc (Takeda Pharmaceuticals); Eli Lilly; Novo Nordisk; Glaxo Smith Kline; Gilead Sciences Inc.; Arrowhead Pharmaceuticals
b. Key factors that are driving the non-alcoholic steatohepatitis clinical trials market growth include rising healthcare expenditure, increasing prevalence of obesity & diabetes mainly due to sedentary lifestyles, and unmet medical needs.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."




