
Metastatic Lung Adenocarcinoma Treatment Market Size, Share & Trends Analysis Report By Treatment (Chemotherapy, Targeted Therapy), By End Use (Hospitals, Specialty Clinics), By Region, And Segment Forecasts, 2025 - 2030
- Report ID: GVR-4-68040-431-1
- Number of Report Pages: 120
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2024
- Forecast Period: 2025 - 2030
- Industry: Healthcare
Market Size & Trends
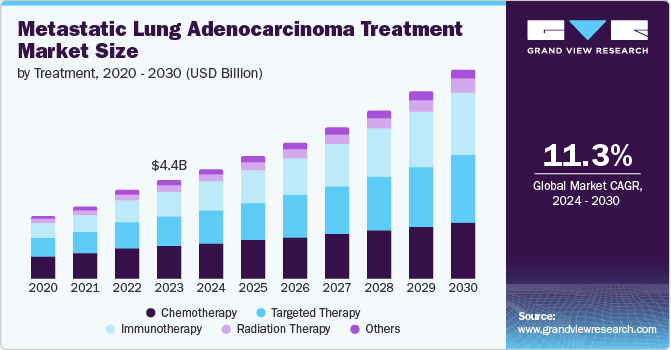
The global metastatic lung adenocarcinoma treatment market size was valued at USD 4.90 billion in 2024 and is expected to grow at a CAGR of 11.3% from 2025 to 2030. This growth is fueled by the rising incidence of lung cancer coupled with advancements in treatment options. As lung cancer rates increase globally, the demand for effective therapies to treat advanced stages of the disease rises.
Recent innovations, including targeted therapies, immunotherapy, and personalized medicine, offer more effective and tailored treatment approaches. These advancements improve survival rates and quality of life for patients, further propelling the market.
Early diagnosis and improved screening methods, such as low-dose CT scans and liquid biopsy, are crucial in detecting metastatic lung adenocarcinoma at earlier stages, leading to better treatment outcomes. As these technologies become more accessible, the demand for targeted therapies and advanced treatments increases. Furthermore, rising awareness and advocacy efforts by healthcare organizations and patient groups have helped educate the public on the importance of early detection and available treatments. This growing awareness is driving more patients to seek timely care, further boosting the metastatic lung adenocarcinoma treatment industry.
Treatment Insights
The chemotherapy segment dominated the market with the largest revenue share of 36% in 2024 due to its well-established efficacy in targeting cancer cells. Chemotherapy remains a primary treatment option, especially in advanced stages of the disease, offering patients significant survival benefits. Its ability to target rapidly dividing cancer cells makes it a crucial part of the treatment regimen. Besides, chemotherapy is often combined with other therapies, such as immunotherapy or targeted therapy, further solidifying its prominent position in the market.
The targeted therapy segment is anticipated to emerge as the fastest-growing segment and register a CAGR of 12.9% from 2025 to 2030, owing to its ability to target specific molecular drivers of cancer precisely. Unlike traditional chemotherapy, targeted therapies focus on genetic mutations or specific proteins that promote cancer cell growth, offering a more personalized and effective treatment approach with fewer side effects. Advances in biomarker testing and the approval of novel targeted agents are fueling this growth. As more patients benefit from targeted therapies, the segment is set to experience rapid expansion in the coming years.
End Use Insights
The hospitals segment recorded the largest market share of 63.9% in 2024, attributed to their ability to offer comprehensive care, advanced diagnostic tools, and access to the latest treatment options. With specialized oncology departments, hospitals provide personalized treatment plans, including chemotherapy, targeted therapies, and immunotherapy, which are critical for managing metastatic lung adenocarcinoma. Moreover, hospitals are equipped with cutting-edge technologies for early detection, leading to improved patient outcomes. Their established infrastructure and collaborations with pharmaceutical companies strengthen their leading position in the market.
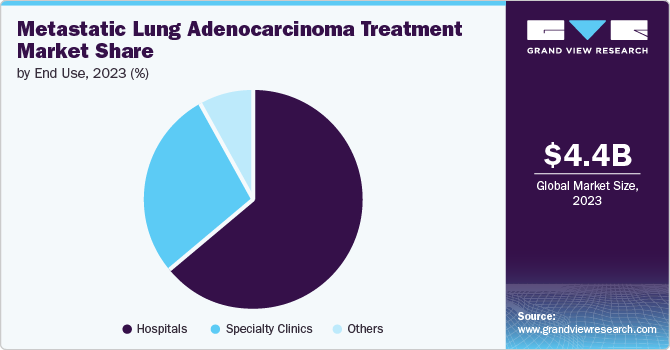
The specialty clinics segment is projected to stand out as the fastest-growing segment and grow at a CAGR of 13.1% from 2025 to 2030, driven by increasing demand for personalized care and targeted therapies. These clinics focus on specific cancer types, offering advanced diagnostic tools, tailored treatment plans, and close patient monitoring. With specialized expertise in lung cancer, specialty clinics can provide cutting-edge therapies, such as immunotherapy and targeted drug treatments, enhancing treatment outcomes. In addition, their patient-centered approach, shorter wait times, and focused care make them an attractive alternative, driving growth in this segment.
Regional Insights
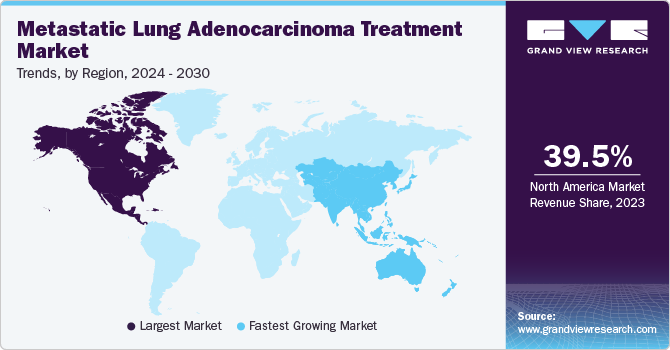
North America metastatic lung adenocarcinoma treatment market secured the largest market share of 39.5% in 2024, propelled by the rising incidence of lung cancer and the growing focus on early detection. Enhanced screening programs and advanced diagnostic tools enable earlier and more accurate detection, improving treatment outcomes and survival rates. As the number of diagnoses continues to rise, particularly in high-risk populations, there is an increasing demand for innovative treatment options. This synergy of early detection and higher case numbers is fueling market growth, with more patients seeking effective therapies for advanced stages of the disease.
U.S. Metastatic Lung Adenocarcinoma Treatment Market Trends
Advancements in targeted therapy and the growing focus on precision medicine are accelerating the expansion of the metastatic lung adenocarcinoma treatment industry across the US. Targeted therapies, which focus on specific genetic mutations in cancer cells, offer more effective and less toxic treatment options. Besides, precision medicine, which tailors treatments based on individual patient profiles, is improving outcomes and reducing side effects. As these innovations evolve, they promise more personalized, efficient, and effective treatments, fueling market expansion and improving survival rates for patients with metastatic lung adenocarcinoma.
The approval of novel therapeutics and the development of combination therapies, are projected to fuel the growth of the Canada metastatic lung adenocarcinoma treatment market. New therapies, such as immune checkpoint inhibitors and targeted treatments, are offering improved survival rates and better quality of life for patients. Furthermore, the growing focus on combining immunotherapy with chemotherapy or targeted therapy enhances treatment efficacy. As these advanced treatment options become more widely accessible, the Canadian market is expected to grow significantly, improving patient outcomes and driving demand for innovative therapies.
Europe Metastatic Lung Adenocarcinoma Treatment Market Trends
Europe is set to expand at the fastest-growing CAGR of 11.1% from 2025 to 2030. The rising focus on personalized and precision medicine is enhancing treatment efficacy for metastatic lung adenocarcinoma in Europe, as therapies are tailored to individual genetic profiles and specific mutations. This approach is driving demand for targeted treatments, improving patient outcomes. Moreover, growing health policy and regulatory support, including faster approval processes by agencies, particularly the European Medicines Agency (EMA), is accelerating access to novel therapies. These advancements and favorable regulatory frameworks are anticipated to expand the Europe metastatic lung adenocarcinoma treatment industry, ensuring better treatment options and broader patient access.
The shift toward immunotherapy and the increase in surgical interventions are expected to propel the market expansion across the UK. Immunotherapy offers targeted, effective treatment options, improving patient outcomes and survival rates, leading to its increased adoption. Also, advancements in surgical techniques and precision medicine are enabling more patients to undergo surgery, further expanding treatment opportunities. This combination of cutting-edge therapies and enhanced surgical approaches is projected to bolster market demand, fostering substantial market growth in the years ahead.
Increasing healthcare investment, improved access to medical services, and a growing emphasis on early detection and screening are set to accelerate the market growth across Germany. Enhanced funding in the healthcare sector is supporting the development of advanced diagnostic tools and innovative treatments. Early detection methods, such as low-dose CT scans, provide timely intervention, improving patient outcomes. As awareness of the importance of screening rises, more patients are being diagnosed earlier, further boosting demand for effective treatments and expanding the market.
Asia Pacific Metastatic Lung Adenocarcinoma Treatment Market Trends
The growing investment in cancer research and the shift toward personalized medicine are expected to fuel the Asia Pacific metastatic lung adenocarcinoma treatment industry. Advancements in targeted therapies, immunotherapy, and precision medicine are revolutionizing treatment approaches, offering patients more effective and tailored options. Increased funding is accelerating the development of innovative treatments, enhancing the potential for better outcomes. As healthcare infrastructure improves and personalized treatments become more accessible, demand for advanced therapies in metastatic lung adenocarcinoma is anticipated to rise, fueling market growth in the region.
Japan is set to grow at a commendable CAGR over the forecast period, owing to growing awareness, patient advocacy, and expanding healthcare access. As awareness campaigns educate the public and healthcare professionals about early detection, more patients are expected to pursue timely treatments, improving outcomes. Patient advocacy groups also push for better therapies and increased access to innovative treatments. In addition, expanded healthcare coverage ensures that more individuals have access to cutting-edge therapies, thereby enhancing treatment adoption and accelerating market growth in Japan.
China is anticipated to achieve a noteworthy share during the forecast period, spurred by regulatory approvals and the rising availability of targeted therapies. As regulatory bodies expedite the approval of innovative treatments, patients gain quicker access to advanced therapies, improving survival rates. The increasing availability of targeted therapies, which offer more personalized and effective treatment options, further fuels demand. These advancements in treatment options, coupled with a supportive regulatory environment, are projected to expand the metastatic lung adenocarcinoma treatment industry in China, improving patient outcomes and market potential.
Key Metastatic Lung Adenocarcinoma Treatment Company Insights
Some of the key companies in the metastatic lung adenocarcinoma treatment market include Pfizer Inc.; Eli Lilly and Company; AstraZeneca; Bristol-Myers Squibb Company; Boehringer Ingelheim International GmbH; Novartis AG; Merck KGaA (EMD Serono); F. Hoffmann-La Roche Ltd; GSK plc.; and AbbVie Inc.
-
Pfizer Inc. specializes in developing and manufacturing innovative medicines and vaccines, focusing on oncology, cardiology, immunology, rare diseases, and infectious diseases. The company is dedicated to advancing healthcare through scientific research and global access to treatments.
-
Eli Lilly and Company offers innovative pharmaceuticals and biotechnology solutions, focusing on treatments for oncology, diabetes, immunology, neurodegenerative diseases, and cardiovascular conditions. Its portfolio includes prescription medications, vaccines, and cutting-edge therapies for various conditions.
Key Metastatic Lung Adenocarcinoma Treatment Companies:
The following are the leading companies in the metastatic lung adenocarcinoma treatment market. These companies collectively hold the largest market share and dictate industry trends.
- Pfizer Inc.
- Eli Lilly and Company
- AstraZeneca
- Bristol-Myers Squibb Company
- Boehringer Ingelheim International GmbH
- Novartis AG
- Merck KGaA (EMD Serono)
- F. Hoffmann-La Roche Ltd
- GSK plc
- AbbVie Inc.
Recent Developments
-
In March 2024, Bristol Myers Squibb's KRYSTAL-12 trial demonstrated that KRAZATI (adagrasib) met its primary endpoint of progression-free survival in patients with pretreated KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer. This result marks a significant step forward in targeted cancer treatment.
-
In February 2024, AstraZeneca's Tagrisso (osimertinib) was approved in the U.S. for chemotherapy treatment in adults with locally advanced or metastatic EGFR-mutated non-small cell lung cancer (NSCLC). This approval provides a comprehensive treatment option, combining targeted therapy and chemotherapy to improve outcomes in advanced disease stages.
Metastatic Lung Adenocarcinoma Treatment Market Report Scope
|
Report Attribute |
Details |
|
Market size value in 2025 |
USD 5.44 billion |
|
Revenue forecast in 2030 |
USD 9.29 billion |
|
Growth Rate |
CAGR of 11.3% from 2025 to 2030 |
|
Base year for estimation |
2024 |
|
Historical data |
2018 - 2024 |
|
Forecast period |
2025 - 2030 |
|
Quantitative units |
Revenue in USD million and CAGR from 2025 to 2030 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Treatment, end use, region |
|
Regional scope |
North America, Europe, Asia Pacific, Latin America, Middle East and Africa |
|
Country scope |
U.S., Canada, Mexico, UK, Germany, France, Spain, Italy, Denmark, Sweden, Norway, Japan, China, India, South Korea, Australia, Thailand, Brazil, Argentina, South Africa, Saudi Arabia, UAE, Kuwait |
|
Key companies profiled |
Pfizer Inc.; Eli Lilly and Company; AstraZeneca; Bristol-Myers Squibb Company; Boehringer Ingelheim International GmbH; Novartis AG; Merck KGaA (EMD Serono); F. Hoffmann-La Roche Ltd; GSK plc.; and AbbVie Inc. |
|
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. Explore purchase options |
Global Metastatic Lung Adenocarcinoma Treatment Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For the purpose of this study, Grand View Research has segmented the global metastatic lung adenocarcinoma treatment market report on the basis of treatment, end use, and region:
-
Treatment Outlook (Revenue, USD Million, 2018 - 2030)
-
Chemotherapy
-
Targeted Therapy
-
Immunotherapy
-
Radiation Therapy
-
Others
-
-
End Use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Specialty Clinics
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Spain
-
Italy
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."




