
Meningitis Diagnostic Testing Market Size, Share & Trends Analysis Report By Type (Latex Agglutination Tests, Lateral Flow Assay), By End Use (Hospitals, Diagnostic Centers), By Region, And Segment Forecasts, 2025 - 2030
- Report ID: GVR-4-68040-009-0
- Number of Report Pages: 120
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
Meningitis Diagnostic Testing Market Trends
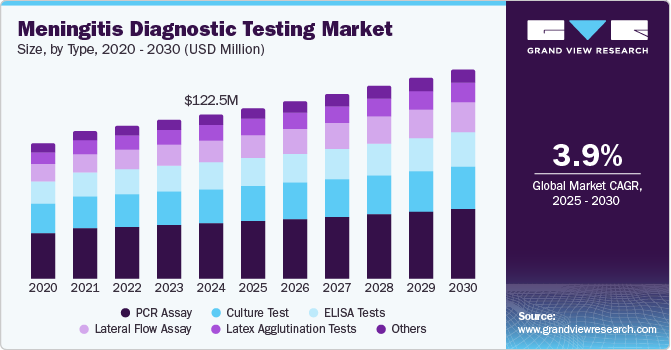
The global meningitis diagnostic testing market size was estimated at USD 122.5 million in 2024 and is projected to grow at a CAGR of 3.9% from 2025 to 2030. The rising incidence of meningitis cases is a significant factor driving the demand for diagnostic tests. Meningitis, which can be caused by bacterial, viral, or fungal infections, affects individuals of all ages, including children and adults. According to a study published in The Lancet, there are approximately 2.51 million new cases of meningitis globally each year, with significant health impacts, including around 236,000 deaths annually. This rising prevalence highlights the urgent need for accurate diagnostic tools to facilitate quick disease identification, essential for timely treatment and improved patient outcomes.
Technological advancements in diagnostic methods have greatly improved the ability to detect meningitis. Techniques such as Polymerase Chain Reaction (PCR), Enzyme-Linked Immunosorbent Assay (ELISA), and rapid antigen testing are widely used due to their enhanced accuracy, speed, and sensitivity. These innovations enable healthcare providers to diagnose meningitis more efficiently, which is crucial in managing this potentially life-threatening condition. The growing adoption of these advanced diagnostic tools in clinical settings further fuels growth in the meningitis diagnostic testing industry, ensuring timely treatment and better patient outcomes.
Meningitis is particularly prevalent among children, especially those under five years of age. This demographic faces a higher risk for severe outcomes from the disease, making early diagnosis essential. Pediatric hospitals and clinics increasingly focus on meningitis diagnosis to ensure prompt treatment for affected children. The rising awareness and emphasis on pediatric care creates a substantial market for diagnostic tests designed for young patients. As healthcare providers recognize the importance of early detection in improving health outcomes for children, the demand for effective diagnostic solutions continues to rise within the meningitis diagnostic testing industry.
Type Insights
The PCR assay segment dominated the market with a revenue share of 33.8% in 2024 due to its high accuracy and rapid turnaround time in diagnosing meningitis. PCR assays can detect specific pathogens in cerebrospinal fluid, which is crucial for timely treatment decisions. The increasing global prevalence of meningitis has heightened the demand for reliable diagnostic methods, making PCR a preferred choice for healthcare providers. In addition, advancements in PCR technology, including real-time capabilities, have improved diagnostic efficiency, further enhancing its market position within the meningitis diagnostic testing industry.
The lateral flow assay segment is projected to grow at the highest CAGR of 5.3% over the forecast period, fueled by its ease of use and rapid results. These assays appeal to point-of-care testing, allowing healthcare professionals to obtain quick diagnoses without complex laboratory procedures. The growing trend toward home-based testing solutions has also contributed to the popularity of lateral flow assays in the meningitis diagnostic testing industry. Furthermore, their cost-effectiveness and ability to deliver results within minutes make them a valuable tool in managing meningitis cases efficiently.
End Use Insights
The hospitals segment dominated the market with the largest revenue share in 2024, attributed to their advanced diagnostic capabilities and high patient volumes. Hospitals typically have access to sophisticated laboratory equipment and trained personnel who can perform complex tests, ensuring accurate diagnosis and effective treatment of meningitis. The increasing incidence of infectious diseases demands rapid and reliable testing within hospital settings, driving growth in this segment. In addition, hospitals serve as primary care facilities where initial evaluations occur, making them integral to the meningitis diagnostic testing industry.
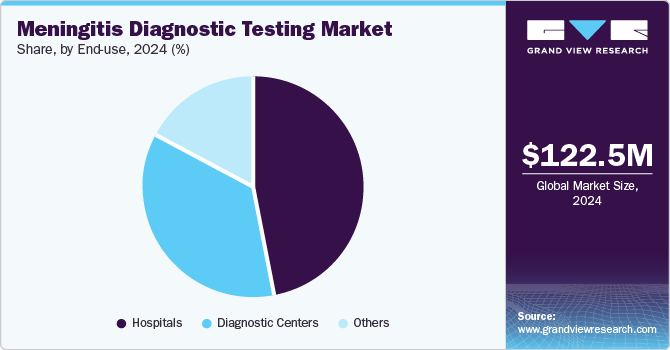
The diagnostic centers segment is projected to grow at a significant CAGR over the forecast period due to the rising demand for specialized testing services. These centers focus on providing comprehensive diagnostic solutions tailored to specific conditions such as meningitis, attracting patients seeking prompt evaluations. The convenience and accessibility offered by diagnostic centers enhance their appeal, particularly as awareness of meningitis symptoms increases. Furthermore, partnerships between hospitals and diagnostic centers facilitate improved service offerings, contributing to meningitis diagnostic testing market growth.
Regional Insights
North America meningitis diagnostic testing market dominated the global with a revenue share of 48.0% in 2024 due to its strong healthcare infrastructure and advanced medical technologies. The region benefits from widespread access to healthcare services and innovative diagnostic tools that enable timely identification of meningitis cases. Increasing public awareness about the disease has led to higher testing rates among healthcare providers and patients. This trend is expected to continue as efforts focus on improving early detection and management strategies within the meningitis diagnostic testing market.
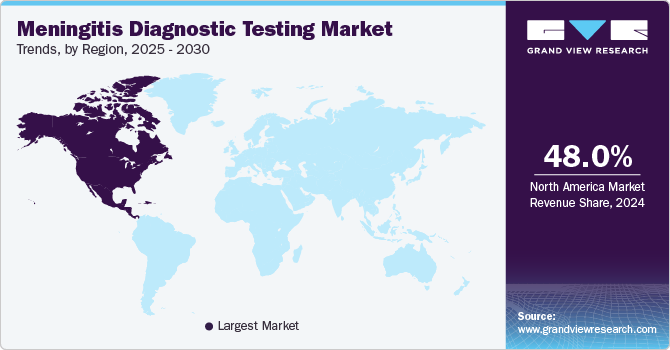
U.S. Meningitis Diagnostic Testing Market Trends
The U.S. meningitis diagnostic testing market dominated North America with a significant revenue share in 2024, fueled by continuous technological innovations. The availability of advanced tests such as PCR assays has transformed how healthcare providers diagnose meningitis, allowing for quicker identification of pathogens. In addition, increased awareness campaigns regarding symptoms associated with meningitis have encouraged more individuals to seek timely medical attention, thereby driving demand for effective diagnostic solutions within this segment.
Europe Meningitis Diagnostic Testing Market Trends
Europe meningitis diagnostic testing market held a substantial market share in 2024, driven by increased investments in healthcare infrastructure and research initiatives to combat infectious diseases. Collaborative efforts among healthcare providers have enhanced diagnostic accuracy and patient outcomes related to meningitis management. Furthermore, ongoing technological advancements are improving existing diagnostics, making them more efficient and reliable within the meningitis diagnostic testing market.
Asia Pacific Meningitis Diagnostic Testing Market Trends
Asia Pacific meningitis diagnostic testing market is expected to register the highest CAGR of 5.8% over the forecast period, which can be attributed to growing awareness about infectious diseases and improvements in healthcare access. The region expanding population coupled with a rising incidence of contagious diseases demands enhanced capabilities for effective disease management through accurate diagnostics. Increased investment from private sectors into healthcare facilities is anticipated further to boost growth within this meningitis diagnostic testing market segment.
The China meningitis diagnostic testing market dominates the Asia Pacific with a significant revenue share in 2024, driven by its rapidly developing healthcare system and increased focus on disease prevention strategies. Enhanced access to advanced diagnostics has improved detection rates for conditions such as meningitis across urban and rural areas. Moreover, public health initiatives aimed at educating citizens about symptoms associated with meningitis contribute to higher testing rates, thus driving growth within this segment of the meningitis diagnostic testing market.
Key Meningitis Diagnostic Testing Company Insights
Some key companies operating in the market include Thermo Fisher Scientific Inc.; Bio-Rad Laboratories, Inc; Abbott.; ELITechGroup; and Seegene Inc. Companies are undertaking strategic initiatives such as mergers, acquisitions, and product launches, to expand their market presence and address the evolving healthcare demands through meningitis diagnostic testing market.
-
Thermo Fisher Scientific Inc. provides various solutions for the meningitis diagnostic testing market, including the Wellcogen Bacterial Antigen Kit, which rapidly detects bacterial antigens from pathogens such as Streptococcus pneumoniae and Neisseria meningitidis. Its product line also features the Wellcogen Neisseria meningitidis B/E. Coli K1 Test Kit and the Wellcogen Neisseria meningitidis A, C, Y, W135 Test Kit, which enhance diagnostic accuracy for different meningococcal groups. These kits are essential for clinical laboratories to ensure timely and effective diagnosis of meningitis.
-
Bio-Rad Laboratories, Inc. provides a range of products for the meningitis diagnostic testing market, focusing on quality control for serology and molecular testing. Its offerings include molecular controls for verification, daily run checks, and serology quality controls designed to challenge assay cutoffs. These products ensure accurate performance across various testing platforms, supporting laboratories in delivering reliable results in the meningitis diagnostic testing market.
Key Meningitis Diagnostic Testing Companies:
The following are the leading companies in the meningitis diagnostic testing market. These companies collectively hold the largest market share and dictate industry trends.
- Thermo Fisher Scientific Inc.
- Bio-Rad Laboratories, Inc
- Abbott.
- ELITechGroup
- Seegene Inc.
- DiaSorin S.p.A.
- CERTEST BIOTEC.
- IMMY.
- Uniogen Oy
- BIOMÉRIEUX
Recent Developments
-
In September 2024, Thermo Fisher Scientific announced the expansion of its bioanalytical laboratory services in Europe. This initiative aimed to enhance its capabilities in supporting the growing demand for bioanalytical testing across various sectors, including pharmaceuticals and biotechnology. The expansion included the addition of state-of-the-art technologies and an increase in laboratory capacity, which positioned the company to serve its clients better and improve turnaround times for critical testing services.
-
In June 2021, Seegene announced a partnership with Bio-Rad to develop and commercialize infectious disease molecular diagnostic products for the U.S. market. This collaboration leveraged high multiplex technology to create diagnostic tests tailored for post-pandemic environments. The partnership marked a significant step for the company in expanding its presence in the U.S., the largest in vitro diagnostics market globally. In addition, the company introduced a new multiplex PCR test capable of screening multiple SARS-CoV-2 variants, further enhancing its product offerings in response to the ongoing public health crisis.
Meningitis Diagnostic Testing Market Report Scope
|
Report Attribute |
Details |
|
Market size value in 2025 |
USD 127.2 million |
|
Revenue forecast in 2030 |
USD 155.9 million |
|
Growth rate |
CAGR of 3.9% from 2025 to 2030 |
|
Base year for estimation |
2024 |
|
Historical data |
2018 - 2023 |
|
Forecast period |
2025 - 2030 |
|
Quantitative units |
Revenue in USD million and CAGR from 2025 to 2030 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, trends |
|
Segments covered |
Type,end use, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country scope |
U.S., Canada, Mexico, UK, Germany, France, Italy, Spain, Norway, Denmark, Sweden,Japan, China, India, South Korea, Australia, Thailand, Brazil, Argentina, South Africa, Saudi Arabia, UAE, Kuwait |
|
Key companies profiled |
Thermo Fisher Scientific Inc.; Bio-Rad Laboratories, Inc; Abbott.; ELITechGroup; Seegene Inc.; DiaSorin S.p.A.; CERTEST BIOTEC.; IMMY.; Uniogen Oy; BIOMÉRIEUX. |
|
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. Explore purchase options |
Global Meningitis Diagnostic Testing Market Report Segmentation
This report forecasts global, regional, and country revenue growth and analyzes the latest industry trends in each sub-segment from 2018 to 2030. For this study, Grand View Research has segmented the global meningitis diagnostic testing market report based on type, end use, and region:
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Latex Agglutination Tests
-
Lateral Flow Assay
-
PCR Assay
-
ELISA Tests
-
Culture Test
-
Others
-
-
End Use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Diagnostic Centers
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Norway
-
Denmark
-
Sweden
-
-
Asia Pacific
-
Japan
-
China
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."




