
Lung Adenocarcinoma Treatment Market Size, Share & Trends Analysis Report By Treatment (Chemotherapy, Immunotherapy, Radiation Therapy), By End-use (Hospitals, Specialty Clinics), By Region (North America, Europe), And Segment Forecasts, 2025 - 2030
- Report ID: GVR-4-68040-428-6
- Number of Report Pages: 100
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
Market Size & Trends
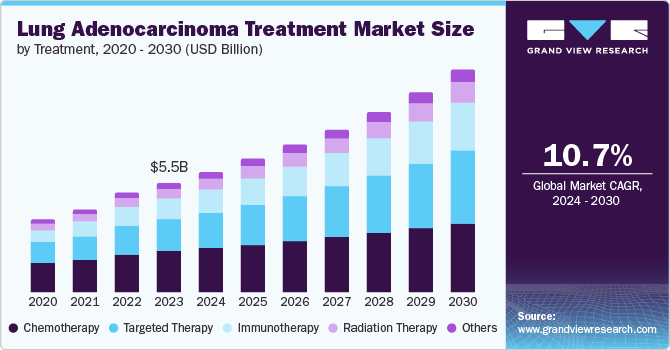
The global lung adenocarcinoma treatment market size was estimated at USD 6.08 billion in 2024 and is projected to grow at a CAGR of 10.7% from 2025 to 2030. The market is driven by several factors, such as the rising number of lung cancer cases, advancements in targeted therapies and immunotherapies, increased awareness and screening efforts, and more investment in research and development. These factors are shaping the treatment options available for patients with this type of lung cancer.
According to WHO, the two most common types of lung cancer are Non-Small Cell Lung Carcinoma (NSCLC) and Small Cell Lung Carcinoma (SCLC). Lung adenocarcinoma, a subtype of NSCLC, develops from the epithelial cells in the lungs.
The rising incidence of lung cancer worldwide is a key factor driving the market. According to the Lung Cancer Research Foundation, an estimated 238,340 individuals in the U.S. were diagnosed with lung cancer in 2023. Over a lifetime, 1 in 16 people develop the disease, affecting 1 in 16 men and 1 in 17 women. Increasing smoking rates in certain regions, along with environmental factors such as air pollution and occupational hazards, have contributed to this trend. With more individuals being diagnosed with lung adenocarcinoma, the demand for effective treatment options continues to grow.
The development of targeted therapies and immunotherapies has played a significant role in market expansion. These treatments have improved patient outcomes with fewer side effects than traditional chemotherapy. Targeted therapies, such as Tyrosine Kinase Inhibitors (TKIs) and Monoclonal Antibodies (mAbs), are designed to attack specific molecules involved in cancer growth and spread. Their effectiveness in treating NSCLC has established them as a standard of care for some patients in the advanced stages of the disease.
Treatment Insights
The chemotherapy segment accounted for the largest revenue share of 36% in 2024. This is primarily due to its established role in lung cancer management and ongoing advancements in treatment regimens. Chemotherapy remains a key treatment approach, particularly for NSCLC. Innovations in this segment focus on developing new drug combinations, less toxic formulations, and improved treatment efficacy while enhancing patient tolerability. In addition, chemotherapy is considered a cost-effective option, making it more accessible to a broader patient population, especially in resource-limited settings.
The immunotherapy segment is expected to witness the fastest growth, with a projected CAGR of 13.5% over the forecast period. The rising prevalence of lung adenocarcinoma has increased the demand for advanced treatment options that offer better efficacy and safety compared to traditional therapies such as chemotherapy and radiation. Immunotherapies, including immune checkpoint inhibitors such as pembrolizumab and nivolumab, have demonstrated significant success in strengthening the body’s immune response against cancer cells. This has led to prolonged survival rates and improved quality of life for patients.
End-use Insights
Hospitals accounted for the largest revenue share of 63.9% in 2024, driven by their comprehensive infrastructure and ability to provide a wide range of advanced cancer treatments. They offer multidisciplinary care, including surgery, chemotherapy, radiation therapy, targeted therapies, and immunotherapies, which are essential for managing complex cases of lung adenocarcinoma. According to the National Center for Biotechnology Information (NCBI), the increasing number of patient admissions for lung cancer treatment is linked to rising incidence rates. In 2023, approximately 609,820 new lung cancer cases were reported in the U.S., contributing to the growth of this segment.
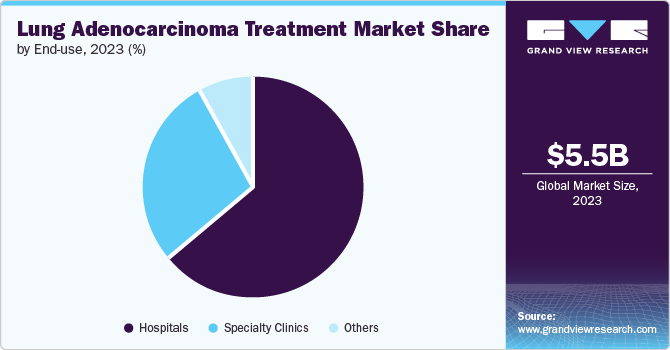
The specialty clinics segment is expected to grow at the fastest CAGR over the forecast period. This growth is driven by the rising demand for personalized and targeted therapies, along with increasing patient awareness of lung cancer treatment options. Specialty clinics focus on specific cancer types, offering tailored treatment plans that integrate the latest advancements, including immunotherapy and targeted therapies. Clinics specializing in lung cancer are also adopting innovative approaches, such as liquid biopsies, which support early detection and treatment monitoring.
Regional Insights
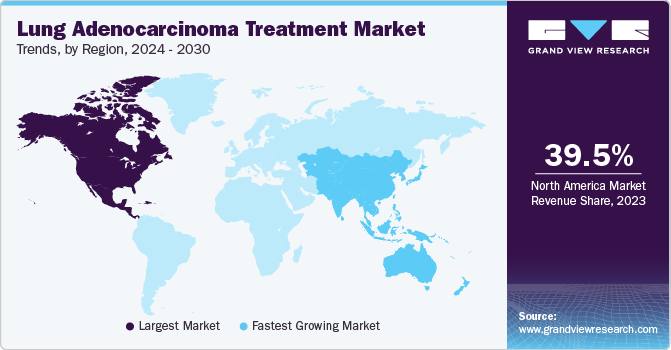
North America lung adenocarcinoma treatment market held the largest revenue share of 39.5% in 2024, driven by the rising incidence of lung adenocarcinoma and a strong focus on innovation in treatment approaches. The increasing prevalence of lung cancer has led to the adoption of more tailored therapies that consider the genetic profile of tumors. The introduction of immune checkpoint inhibitors and targeted agents such as EGFR inhibitors has significantly transformed treatment protocols. In addition, pharmaceutical companies in the Lung Adenocarcinoma Treatment industry are engaging in mergers and acquisitions to expand their product portfolios and strengthen research capabilities.
U.S. Lung Adenocarcinoma Treatment Market Trends
The U.S. lung adenocarcinoma treatment market led North America in 2024, driven by rapid innovation and a highly competitive landscape. Strategic mergers and acquisitions among pharmaceutical companies have contributed to the expansion of treatment options, while regulatory support, such as the FDA’s accelerated approval pathways, has enabled faster access to promising therapies for patients with advanced disease stages.
In December 2024, the FDA granted accelerated approval to zenocutuzumab-zbco (Bizengri) for adults with advanced, unresectable, or metastatic NSCLC harboring a neuregulin 1 (NRG1) gene fusion, following disease progression on prior systemic therapy. These developments reflect the evolving landscape of lung adenocarcinoma treatment, with continued efforts to introduce targeted and effective therapies.
Asia Pacific Lung Adenocarcinoma Treatment Market Trends
Asia Pacific lung adenocarcinoma treatment market is expected to register the fastest CAGR of 11.4% during the forecast period. The industry is experiencing significant growth due to the rising prevalence of lung cancer, particularly among non-smokers. Innovative therapies, including targeted and immunotherapies, are gaining traction, with drugs such as osimertinib and nivolumab playing a key role in treatment advancements.
The increasing adoption of personalized medicine is another key trend, as genetic profiling becomes more accessible, enabling tailored treatment approaches that improve patient outcomes. Regulatory agencies in countries such as Australia and South Korea are streamlining approval processes for new treatments, supporting market entry, and expanding treatment availability across the region.
China Lung Adenocarcinoma Treatment Market Trends
China lung adenocarcinoma treatment market dominated the Asia Pacific Lung Adenocarcinoma Treatment industry in 2024, driven by a high incidence of lung cancer and a growing emphasis on innovative therapies. Recent regulatory approvals for targeted treatments, such as Anaplastic Lymphoma Kinase (ALK) inhibitors and immunotherapies, have expanded patient options, supporting market growth.
The increasing prevalence of smoking and environmental pollution continues to contribute to the rising lung cancer burden. In addition, the market is witnessing a surge in mergers and acquisitions among pharmaceutical companies, aiming to strengthen product portfolios and enhance research capabilities to meet the growing demand for effective treatment options.
Middle East & Africa Lung Adenocarcinoma Treatment Market Trends
The Middle East & Africa lung adenocarcinoma treatment market is experiencing notable growth, driven by the rising incidence of lung cancer, advancements in targeted therapies, and increasing awareness of early detection. Factors such as smoking and environmental pollutants have contributed to a higher number of cases, leading to greater demand for effective treatments. Developments in targeted therapies and immunotherapies have improved patient outcomes, while collaborations between healthcare providers and pharmaceutical companies are enhancing access to advanced treatments. Educational initiatives for healthcare professionals are also supporting the adoption of innovative therapies. Countries such as South Africa, the United Arab Emirates, and Saudi Arabia are leading these advancements with investments in healthcare infrastructure and training programs. Moreover, with growing awareness and accessibility, the lung adenocarcinoma treatment industry in the region is expected to expand further.
Key Lung Adenocarcinoma Treatment Company Insights
Some of the key companies operating in the lung adenocarcinoma treatment industry are Pfizer Inc.; AstraZeneca; and Bristol-Myers Squibb Company. These companies are expanding their market presence by launching new products, collaborating, and adopting various other strategies.
-
Pfizer Inc. offers targeted therapies and immunotherapies to improve patient outcomes. The company focuses on precision medicine, developing treatments based on genetic mutations and biomarkers. Through ongoing research, clinical trials, and strategic collaborations, Pfizer continues to expand its oncology portfolio and enhance treatment options for lung adenocarcinoma.
-
AstraZeneca focuses on targeted therapies and immunotherapies designed to improve treatment efficacy. The company has developed innovative drugs, including EGFR inhibitors and immune checkpoint inhibitors, to address different stages of lung adenocarcinoma. With a strong emphasis on research, clinical advancements, and strategic collaborations, AstraZeneca continues to expand its oncology portfolio to enhance patient care.
Key Lung Adenocarcinoma Treatment Companies:
The following are the leading companies in the lung adenocarcinoma treatment market. These companies collectively hold the largest market share and dictate industry trends.
- Pfizer Inc.
- AstraZeneca
- Bristol-Myers Squibb Company
- Eli Lilly and Company
- Boehringer Ingelheim International GmbH
- Novartis AG
- Merck KGaA (EMD Serono)
- AbbVie Inc.
- Astellas Pharma Inc.
- F. Hoffmann-La Roche Ltd
View a comprehensive list of companies in the Lung Adenocarcinoma Treatment Market
Recent Developments
-
In April 2024, the FDA approved alectinib (Alecensa) as an adjuvant for patients diagnosed with ALK-positive NSCLC after tumor resection. An FDA-sanctioned test confirmed the condition.
-
In March 2024, Pfizer Inc. released long-term follow-up results from the Phase 3 CROWN trial, which evaluated the efficacy of LORBRENA (lorlatinib), a third-generation ALK inhibitor, compared to XALKORI (crizotinib) in patients with untreated, ALK-positive advanced NSCLC.
Lung Adenocarcinoma Treatment Market Report Scope
|
Report Attribute |
Details |
|
Market size value in 2025 |
USD 6.72 billion |
|
Revenue forecast in 2030 |
USD 11.18 billion |
|
Growth rate |
CAGR of 10.7% from 2025 to 2030 |
|
Base year for estimation |
2024 |
|
Historical data |
2018 - 2023 |
|
Forecast period |
2025 - 2030 |
|
Quantitative units |
Revenue in USD million/billion and CAGR from 2025 to 2030 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, trends |
|
Segments covered |
Treatment, end-use, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country scope |
U.S., Canada, Mexico, UK, Germany, France, Italy, Spain, Denmark, Sweden, Norway, China, Japan, India, Australia, South Korea, Thailand, Brazil, Argentina, South Africa, Saudi Arabia, UAE, Kuwait |
|
Key companies profiled |
Pfizer Inc.; AstraZeneca; Bristol-Myers Squibb Company; Eli Lilly and Company; Boehringer Ingelheim International GmbH; Novartis AG; Merck KGaA (EMD Serono); AbbVie Inc.; Astellas Pharma Inc.; F. Hoffmann-La Roche Ltd. |
|
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. Explore purchase options |
Global Lung Adenocarcinoma Treatment Market Report Segmentation
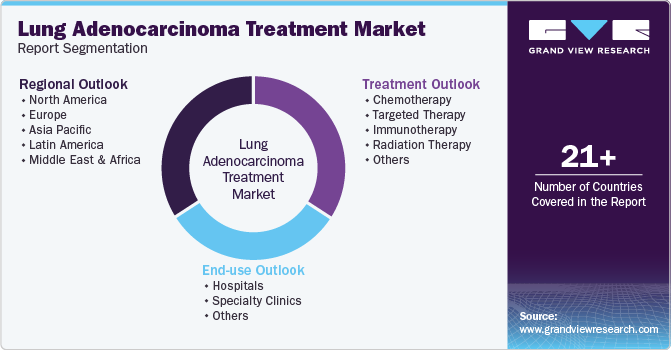
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global Lung Adenocarcinoma Treatment Market report based on treatment, end-use, and region:
-
Treatment Outlook (Revenue, USD Million, 2018 - 2030)
-
Chemotherapy
-
Targeted Therapy
-
Immunotherapy
-
Radiation Therapy
-
Others
-
-
End-use Channel Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Specialty Clinics
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."




