- Home
- »
- Pharmaceuticals
- »
-
Irritable Bowel Syndrome Treatment Market Size Report 2030GVR Report cover
![Irritable Bowel Syndrome Treatment Market Size, Share & Report]()
Irritable Bowel Syndrome Treatment Market Size, Share & Analysis Report By Type (IBS-C, IBS-D), By Product (Xifaxan, Linzess/Constella, Viberzi, Amitiza), By Distribution Channel, By Region, And Segment Forecasts, 2025 - 2030
- Report ID: GVR-3-68038-319-5
- Number of Report Pages: 100
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
Market Size & Trends
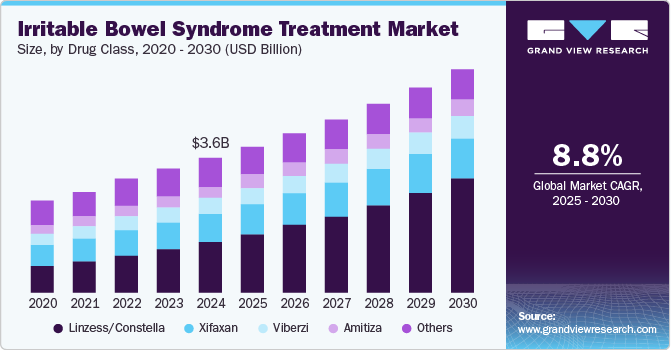
The global irritable bowel syndrome treatment market size was valued at USD 3.64 billion in 2024 and is anticipated to grow at a CAGR of 8.8% from 2025 to 2030. The market growth is attributed to the increasing prevalence of irritable bowel syndrome (IBS), influenced by stress and unhealthy diets. In addition, the increased awareness about IBS and its treatment options encourages more patients to seek care. In addition, advancements in drug development, particularly for constipation-predominant IBS, and the commercialization of existing therapies such as linzess contribute significantly to market expansion.
Irritable bowel syndrome (IBS) is a prevalent stomach condition that impacts the large intestine. It manifests through various symptoms, including abdominal discomfort, bloating, gas, cramping, and irregular bowel habits, which can range from constipation to diarrhea. IBS is categorized into three primary types: IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), and mixed IBS, which features alternating symptoms of both. Management of IBS typically involves lifestyle modifications such as stress reduction, dietary changes, and appropriate medical treatments.
In addition, available medications for IBS include rifaximin, eluxadoline, lubiprostone, and linaclotide. Antispasmodics such as dicyclomine, peppermint oil, and hyoscyamine are commonly utilized to alleviate symptoms. The treatment approach is often tailored to the individual's specific type of IBS and symptom profile. Patients are encouraged to work closely with healthcare providers to develop a personalized management plan that addresses their unique challenges and improves their quality of life. Understanding the nuances of IBS can lead to more effective treatment strategies and better outcomes for those affected by this chronic condition.
The increasing global elderly population is positively impacting the IBS treatment market. Furthermore, numerous clinical trials, including fecal microbiota transplantation (FMT), are underway to enhance IBS therapies, further driving the market growth. Moreover, as part of the Sustainable Development Goals (SDGs), various countries' regulatory bodies have committed to significantly boosting investments in research and development (R&D) initiatives and increasing the number of researchers by 2030.
Type Insights
The IBS-D segment dominated the market with the largest revenue share of 49.8% in 2024 attributed to a rise in diagnosed cases and an increasing understanding of the condition's impact on quality of life. The effectiveness of treatments such as Viberzi (eluxadoline) has led to higher prescription rates among healthcare providers. In addition, ongoing clinical trials and research into innovative therapies are expected to enhance treatment options for IBS-D. Furthermore, the growing emphasis on patient education and awareness campaigns also plays a crucial role in encouraging individuals to seek appropriate care, thereby expanding the market for IBS-D treatments.
The IBS-C segment is expected to grow at a CAGR of 11.9% over the forecast period, owing to the increasing prevalence of this condition and the rising adoption of effective medications such as Linzess (linaclotide) and Amitiza (lubiprostone). In addition, the growing awareness surrounding IBS-C and educational initiatives that encourage patients to seek treatment significantly boosts market demand. Furthermore, advancements in drug development and the introduction of new therapies are expected to drive further growth in this segment, addressing unmet needs and improving patient outcomes.
Drug Class Insights
Linzess/Constella dominated the market and accounted for the largest revenue share of 37.3% in 2024 attributed to its effectiveness in managing constipation-predominant IBS (IBS-C). The drug has gained significant adoption across major markets, including the U.S., Europe, and Japan, due to its ability to alleviate abdominal pain and improve bowel movement frequency. Furthermore, the recent approval of Linzess in China has expanded its market reach. Increased awareness and educational campaigns about IBS encourage more patients to seek treatment, further boosting segment growth.
Viberzi is expected to grow at a CAGR of 7.5% over the forecast period, contributing to the growth of the IBS treatment market, particularly for diarrhea-predominant IBS (IBS-D). Its unique mechanism of action helps regulate bowel function and reduce abdominal discomfort, making it a preferred choice for many patients. In addition, ongoing clinical trials and research initiatives aimed at enhancing IBS treatments are expected to solidify Viberzi's position in the market. Furthermore, the increasing prevalence of IBS and heightened awareness among healthcare providers about available treatment options are other factors driving Viberzi's growth.
Distribution Channel Insights
Hospital pharmacies led the market and accounted for the largest revenue share in 2024, which is attributed to their comprehensive infrastructure and integrated patient care systems. In addition, hospital pharmacies provide a wide range of medications, including specialized treatments for IBS, which enhances patient trust and adherence to prescribed therapies. The ability to offer personalized care and immediate access to healthcare professionals facilitates effective diagnosis and management of IBS. Furthermore, the increasing incidence of gastrointestinal disorders among hospitalized patients drives demand for IBS treatments, further solidifying the role of hospital pharmacies in this market.
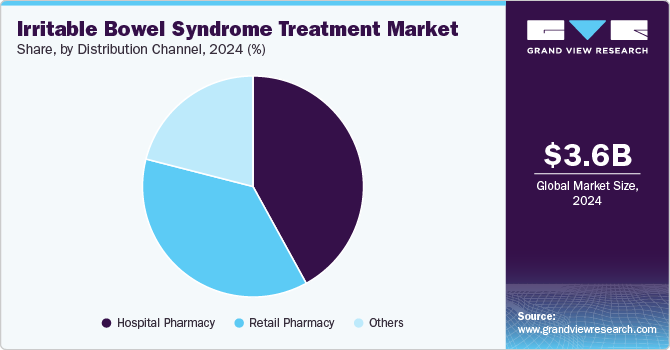
Retail pharmacies are expected to grow at a CAGR of 9.2% over the forecast period, owing to their accessibility and patient convenience. They are essential distribution points for prescription and OTC medications, making IBS treatments available to a wider patient pool. In addition, the personalized service offered by retail pharmacists enhances patient education and compliance with treatment regimens. Furthermore, the rising awareness of IBS and its treatment options encourages more individuals to seek assistance at retail pharmacies. Moreover, expanding product offerings, including dietary supplements and probiotics, are expected to boost the retail pharmacy segment's growth in the coming years.
Regional Insights
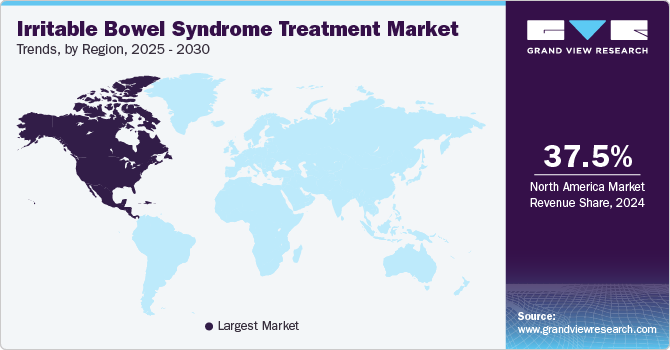
North America irritable bowel syndrome (IBS) treatment market dominated the global market and accounted for the largest revenue share of 37.5% in 2024 attributed to the high prevalence of IBS and the increasing awareness of its management options. In addition, introducing innovative therapies and personalized treatment plans enhances patient outcomes, further propelling market expansion. Furthermore, the presence of major pharmaceutical companies and ongoing research initiatives also contribute to the robust growth in this region.
U.S. Irritable Bowel Syndrome Treatment Market Trends
The irritable bowel syndrome treatment market in the U.S. dominated North America, with the largest revenue share in 2024, significantly influenced by the rising demand for effective medications and therapies. In addition, the growing awareness among patients about IBS and its treatment options has led to increased consultations with healthcare providers. Furthermore, advancements in drug development, including new formulations and delivery methods, are enhancing treatment efficacy. Moreover, integrating dietary management strategies and lifestyle modifications into treatment plans is gaining traction, contributing to a holistic approach that improves patient satisfaction and adherence to therapy.
Asia Pacific Irritable Bowel Syndrome Treatment Market Trends
Asia Pacific irritable bowel syndrome treatment market is expected to grow at a CAGR of 9.8% over the forecast period, attributed to increasing awareness about gastrointestinal disorders. In addition, the rising prevalence of IBS, coupled with improved healthcare access and infrastructure, is driving demand for effective treatments. Furthermore, dietary strategies are becoming more recognized as essential components of IBS management, leading to a surge in probiotic and dietary supplement sales. Moreover, the growing middle-class population with higher disposable incomes also facilitates greater access to healthcare services.
The irritable bowel syndrome treatment market in Japan is expected to grow significantly, owing to an aging population and increased recognition of gastrointestinal health issues. The government’s focus on improving healthcare services and promoting awareness about IBS has led to more patients seeking diagnosis and treatment. In addition, innovative therapies tailored to Japanese patients’ needs are being introduced, enhancing treatment options available in the market. Furthermore, the collaboration between pharmaceutical companies and healthcare providers fosters better management strategies for IBS patients.
Europe Irritable Bowel Syndrome Treatment Market Trends
The growth of Europe irritable bowel syndrome treatment market is driven by increasing awareness campaigns aimed at destigmatizing gastrointestinal disorders. Countries such as Germany and the UK are seeing increased patient engagement regarding IBS symptoms and available treatments. In addition, established healthcare systems facilitate access to innovative therapies, while ongoing clinical research contributes to developing new medications. Furthermore, there is a growing trend toward integrating lifestyle changes and dietary modifications into treatment plans, positively impacting patient outcomes across the region.
Key Irritable Bowel Syndrome Treatment Company Insights
Some key players in the market are Ironwood Pharmaceuticals, Inc., Allergan, Astellas Pharma, Inc., and others. These companies employ various strategies to gain a competitive edge, such as launching new products to cater to diverse patient needs, focusing on personalized medicine to enhance treatment efficacy, and leveraging emerging markets to tap into increasing awareness about IBS. Furthermore, these businesses engage in collaborative research initiatives with healthcare organizations to drive innovation while integrating digital health solutions for enhanced patient access and adherence.
-
Ironwood Pharmaceuticals, Inc. specializes in developing and commercializing innovative therapies for gastrointestinal disorders. Its flagship product, LINZESS, is a first-in-class medication approved for the treatment of constipation-predominant irritable bowel syndrome (IBS-C) and chronic idiopathic constipation (CIC). The company focuses on advancing gastrointestinal health through targeted drug development. It has recently expanded LINZESS indications to include pediatric patients aged 6-17 years with functional constipation, enhancing its therapeutic offerings in the GI segment.
-
AstraZeneca manufactures various medications across various therapeutic areas, including gastrointestinal health. The company's portfolio encompasses treatments for oncology, cardiovascular diseases, respiratory conditions, and rare diseases. AstraZeneca extensively contributes to advancements in IBS management through its research capabilities and partnerships with other institutes that enhance access to effective therapies.
Key Irritable Bowel Syndrome Treatment Companies:
The following are the leading companies in the irritable bowel syndrome treatment market. These companies collectively hold the largest market share and dictate industry trends.
- Ironwood Pharmaceuticals, Inc.
- Allergan
- Astellas Pharma, Inc.
- Takeda Pharmaceutical Company Limited
- AstraZeneca
- Sanofi S.A.
- Sebela Pharmaceuticals Inc.
- Bausch Health
- Synthetic Biologics, Inc.
- Ardelyx
Recent Developments
-
In June 2023, Ironwood Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) approved a new indication for LINZESS (linaclotide), allowing its use in treating functional constipation in pediatric patients aged 6 to 17 years. This approval expanded LINZESS's role in the IBS treatment market, where it is already effective for adults with constipation-predominant irritable bowel syndrome (IBS-C). The decision is based on positive clinical trial results, providing an important option for children suffering from functional constipation and enhancing their quality of life.
-
In October 2023, Sanofi and Teva Pharmaceuticals partnered to develop a novel anti-TL1A therapy, TEV’574, to treat inflammatory bowel diseases, precisely ulcerative colitis and Crohn's disease. This collaboration highlighted both companies' commitment to addressing unmet needs in the IBS treatment market, with TEV’574 in clinical trials.
Irritable Bowel Syndrome Treatment Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 3.96 billion
Revenue forecast in 2030
USD 6.02 billion
Growth rate
CAGR of 8.8% from 2025 to 2030
Base year for estimation
2024
Historical data
2018 - 2023
Forecast period
2025 - 2030
Quantitative units
Revenue in USD million/billion and CAGR from 2025 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Type, drug class, distribution channel, region
Regional scope
North America, Europe, Asia Pacific, Latin America, MEA
Country scope
U.S., Canada, Germany, UK, France, Italy, Spain, China, Japan, Brazil, South Africa
Key companies profiled
Ironwood Pharmaceuticals, Inc.; Allergan; Astellas Pharma, Inc.; Takeda Pharmaceutical Company Limited; AstraZeneca; Sebela Pharmaceuticals Inc.; Bausch Health; Synthetic Biologics, Inc.; Ardelyx
Customization scope
Free report customization (equivalent to 8 analyst working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Irritable Bowel Syndrome Treatment Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and analyzes the latest industry trends in each sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global irritable bowel syndrome treatment market report based on type, drug class, distribution channel, and region.
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
IBS-C
-
IBS-D
-
-
Drug Class Outlook (Revenue, USD Million, 2018 - 2030)
-
Xifaxan
-
Linzess/Constella
-
Viberzi
-
Amitiza
-
Others
-
-
Distribution Channel Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospital Pharmacy
-
Retail Pharmacy
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
-
Asia Pacific
-
China
-
Japan
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
-
Share this report with your colleague or friend.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities. Contact us now
![Certified Icon]()
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."





