
Human Metapneumovirus Diagnostics Market Size, Share & Trends Analysis Report By Technology (PCR Based Diagnostics, Microarray Technology), By End-use (Hospitals & Clinics, Diagnostic & Reference Laboratories), By Region, And Segment Forecasts, 2025 - 2030
- Report ID: GVR-4-68040-497-3
- Number of Report Pages: 160
- Format: PDF
- Historical Range: 2018 - 2024
- Forecast Period: 2025 - 2030
- Industry: Healthcare
HMPV Diagnostics Market Size & Trends
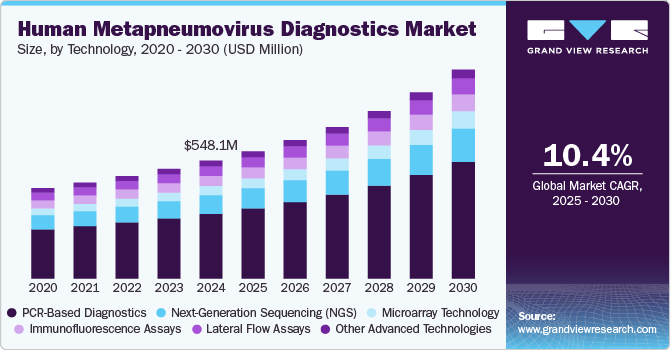
The global human metapneumovirus diagnostics market size was estimated at USD 548.11 million in 2024 and is expected to grow at a CAGR of 10.43% from 2025 to 2030. The Human Metapneumovirus (hMPV) diagnostics market is growing due to the rising prevalence of respiratory infections globally and the increasing awareness of hMPV as a significant cause of these illnesses. hMPV is particularly prevalent among vulnerable populations, such as young children, the elderly, and immunocompromised individuals. Studies estimate that hMPV accounts for 5-10% of hospitalizations for acute respiratory infections in children under five and contributes to severe cases in older adults. Seasonal outbreaks and the overlapping symptoms with other respiratory viruses, like influenza and RSV, are driving the need for precise and timely diagnostics.
The rising prevalence of respiratory infections is a major driver of growth in the Human Metapneumovirus (hMPV) diagnostics industry. hMPV, a leading cause of acute respiratory infections, particularly affects vulnerable groups such as young children, the elderly, and immunocompromised individuals. Studies have shown that hMPV is responsible for a significant percentage of hospitalizations related to respiratory illnesses, especially during seasonal outbreaks. The growing burden of respiratory diseases worldwide, coupled with increased awareness of hMPV’s role, has led to heightened demand for diagnostic tools that can accurately differentiate it from other viral infections like RSV and influenza.
Advancements in diagnostic technologies, particularly the development of PCR-based assays and multiplex testing platforms, are fueling market growth. These advanced diagnostic methods offer high sensitivity, accuracy, and the ability to detect multiple pathogens simultaneously, enabling faster and more reliable diagnosis of hMPV infections. The integration of automation and digital technologies into diagnostic workflows has further enhanced efficiency, making these tools more appealing to healthcare providers. Continuous research and innovation by key players in the market are resulting in the introduction of novel diagnostic solutions, driving adoption and market expansion.
In addition, increasing awareness of respiratory diseases and their potential complications has led to a growing focus on early and accurate diagnosis, contributing to market growth. Governments and healthcare organizations worldwide are investing in improving diagnostic capabilities, particularly in regions with a high prevalence of respiratory infections. For example, public health initiatives aimed at enhancing disease surveillance and early detection are encouraging the adoption of advanced diagnostic tools. In addition, rising healthcare expenditure in emerging economies is expanding access to high-quality diagnostics, further supporting market growth.
However, the high cost associated with advanced diagnostic technologies, such as PCR-based assays and multiplex platforms, is a significant restraint for the market growth. These tools, while highly accurate and efficient, often require specialized equipment, trained personnel, and substantial infrastructure, making them less accessible in low-resource settings and emerging economies. Additionally, the lack of awareness about hMPV among healthcare providers and patients in some regions contributes to underdiagnosis and limited adoption of diagnostics. Regulatory hurdles and lengthy approval processes for new diagnostic tools further impede market growth, as they delay product availability and increase development costs. These challenges highlight the need for affordable, scalable, and easily accessible diagnostic solutions to ensure broader market penetration and address the global burden of hMPV infections effectively.
Market Concentration & Characteristics
The degree of innovation in the Human Metapneumovirus (hMPV) diagnostics industry is high, driven by the need for faster, more accurate, and cost-effective diagnostic solutions. Continuous advancements in PCR-based assays, multiplex testing, and point-of-care devices have significantly improved early detection and diagnosis. Companies are also exploring novel technologies like CRISPR-based diagnostics and biosensors to enhance the sensitivity and specificity of tests. These innovations aim to meet the growing demand for precise and rapid detection, reducing diagnostic time and improving patient outcomes. The market is witnessing a surge in research and development activities, with several diagnostic companies constantly upgrading their products.
The level of mergers and acquisitions (M&A) activities in the hMPV diagnostics industry is medium. While large diagnostics companies like Abbott Laboratories, Roche, and Thermo Fisher Scientific engage in strategic acquisitions to expand their product portfolios, the market remains relatively fragmented with numerous smaller players. These acquisitions often focus on enhancing research capabilities, acquiring novel technologies, or expanding market reach. Partnerships and collaborations are also common, particularly with diagnostic companies aiming to integrate cutting-edge technologies, such as multiplex molecular assays, into their portfolios.
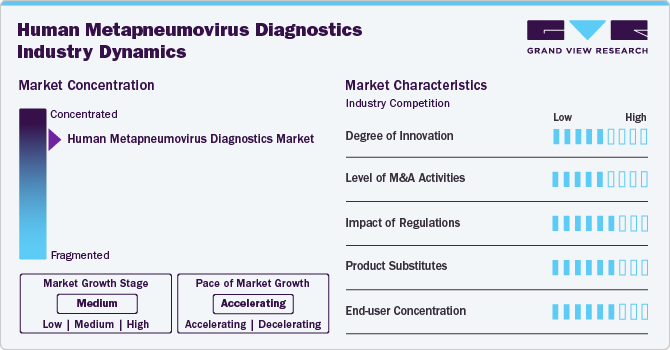
The impact of regulations on the hMPV diagnostics industry is high. Strict regulatory standards set by organizations like the U.S. FDA and the European Medicines Agency (EMA) govern the approval and commercialization of diagnostic tests. These regulations ensure that diagnostic devices meet safety, quality, and performance standards before reaching the market. Compliance with these stringent regulations is crucial for companies to maintain consumer trust and avoid legal challenges. However, the approval process can be lengthy and costly, which can slow down product launches and limit market entry for smaller players.
The threat of product substitutes in the hMPV diagnostics industry is low. PCR-based and other molecular diagnostic tests are currently the most accurate and widely used methods for detecting hMPV infections. While there are alternatives like antigen-based tests and rapid diagnostic tests, they often lack the sensitivity and accuracy of molecular methods, especially in detecting low viral loads. Moreover, advancements in PCR technology have made it more accessible and cost-effective, limiting the need for substitutes.
The end-user concentration in the hMPV diagnostics industry is medium. The primary end-users include hospitals, diagnostic and reference laboratories, and outpatient clinics. Diagnostic laboratories are seeing significant demand due to their ability to perform specialized and advanced tests, which makes them a major end-user in the market. Hospitals and healthcare institutions also contribute substantially, especially in regions with higher prevalence rates of respiratory infections.
Technology Insights
PCR-based diagnostics segment dominated the overall market with largest revenue share of 54.97% in 2024. The dominance of the segment can be attributed to its high sensitivity, accuracy, and ability to detect low viral loads in respiratory specimens. PCR assays are widely regarded as the gold standard for diagnosing hMPV infections, as they can provide definitive results even in early stages of infection. This method enables rapid detection of multiple pathogens simultaneously, improving the efficiency of diagnosis in clinical settings. The growing adoption of PCR-based diagnostics is driven by advancements in technology, making PCR more cost-effective and accessible. Additionally, the increasing prevalence of respiratory infections, especially in vulnerable populations such as young children, the elderly, and immunocompromised individuals, has heightened the need for accurate and quick diagnostic tools. As healthcare providers seek reliable methods for managing hMPV infections, the demand for PCR-based diagnostics continues to rise, contributing significantly to the growth of this segment in the market.
Microarray technology segment is expected to grow at the fastest growth rate from 2025 to 2030 due to its ability to perform high-throughput, multiplex testing, allowing for the simultaneous detection of multiple pathogens, including hMPV, in a single sample. This technology offers significant advantages over traditional methods, such as PCR, by providing a broader diagnostic scope and more comprehensive data. As the need for rapid, accurate, and cost-effective diagnostic tools increases, particularly in environments with high patient volumes, microarray technology has gained traction. The ability to detect a range of respiratory viruses, including hMPV, with a single test is particularly valuable in managing outbreaks and ensuring targeted treatment.
End-use Insights
Hospitals and clinics segment held the largest revenue share of 50.62% in 2024. The growth of the segment is attributed to the critical role of hospitals and clinics in diagnosing and treating respiratory infections in both inpatient and outpatient settings. These healthcare facilities are the primary points of care for patients presenting with symptoms of viral infections, including those caused by hMPV. The high volume of patient visits, especially during peak respiratory infection seasons, drives the demand for accurate and rapid diagnostic tests. Hospitals and clinics rely heavily on advanced diagnostic technologies, such as PCR-based assays and microarrays, to identify hMPV infections promptly and initiate appropriate treatment.
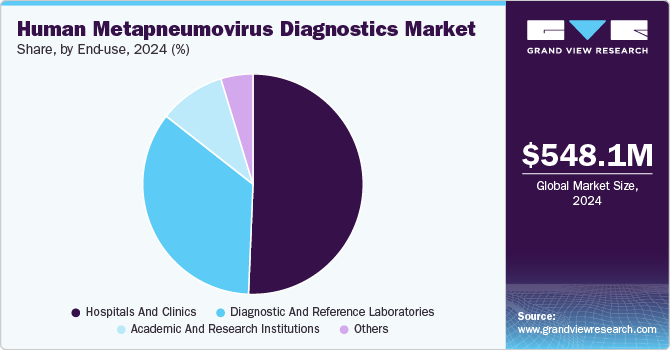
Diagnostic and reference laboratories are anticipated to grow at fastest growth rate from 2025 to 2003 due to their pivotal role in providing specialized, accurate, and high-throughput testing services. These laboratories are equipped with advanced technologies, such as PCR-based assays and microarray platforms, which enable them to perform complex diagnostic tests with high sensitivity and precision. As the demand for rapid and accurate hMPV diagnostics rises, particularly for high-risk populations like the elderly, children, and immunocompromised individuals, these laboratories are positioned to meet the need for comprehensive testing. Additionally, the increasing trend of outsourcing diagnostic services to specialized labs further supports the growth of this segment.
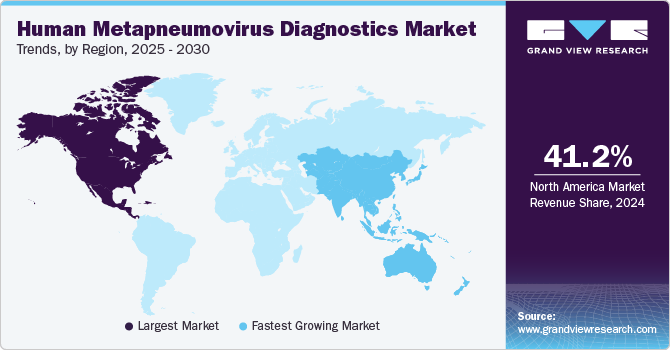
Regional Insights
The North America human metapneumovirus diagnostics market dominated the overall global market and accounted for the 41.18% share in 2024, driven by a combination of factors including advanced healthcare infrastructure, high prevalence of respiratory infections, and increased demand for rapid and accurate diagnostic solutions. In North America, healthcare facilities such as hospitals and diagnostic laboratories are adopting cutting-edge technologies like PCR assays and multiplex testing to detect hMPV infections efficiently. The aging population, as well as the rise in immunocompromised individuals, contributes to the heightened demand for diagnostic services to manage respiratory illnesses.
U.S. Human Metapneumovirus Diagnostics Market Trends
The U.S. Human Metapneumovirus (hMPV) diagnostics market held a significant share of North America market in 2024, driven by the advanced healthcare system, high healthcare expenditure, and a growing focus on early and accurate detection of respiratory infections. The widespread use of PCR-based assays, multiplex testing, and other advanced diagnostic technologies in U.S. hospitals, clinics, and diagnostic laboratories is accelerating the adoption of hMPV diagnostic tools. With the increasing prevalence of viral infections, particularly among vulnerable populations such as children, the elderly, and those with weakened immune systems, the demand for rapid, precise testing is at an all-time high.
Europe Human Metapneumovirus Diagnostics Market Trends
The Human Metapneumovirus (hMPV) diagnostics market in the Europe is experiencing significant growth. The growth is supported by increasing awareness of respiratory infections and the need for timely, accurate diagnostic solutions. The country’s well-established healthcare systems and widespread adoption of advanced diagnostic technologies, such as PCR-based tests and multiplex assays, contribute to the rising demand for efficient hMPV diagnostics. Furthermore, the growing focus on managing infectious diseases and the rise in immunocompromised and elderly populations in Europe are fueling the demand for reliable diagnostic solutions.
The UK Human Metapneumovirus (hMPV) diagnostics market is experiencing significant growthprimarily due to an increasing emphasis on early detection and effective management of respiratory infections. The prevalence of respiratory diseases, particularly during seasonal outbreaks, has heightened the need for rapid and reliable diagnostic tools in hospitals, clinics, and diagnostic laboratories.
The Human Metapneumovirus (hMPV) diagnostics market in Germany is experiencing significant growth, driven by the country’s advanced healthcare system, increasing incidence of respiratory infections, and a strong focus on early and precise diagnostics. The country’s robust healthcare infrastructure, supported by public and private healthcare providers, ensures widespread access to high-quality diagnostic services.
Asia Pacific Human Metapneumovirus Diagnostics Market Trends
The Asia Pacific Human Metapneumovirus (hMPV) diagnostics market is experiencing the fastest growth driven by rising healthcare awareness, improving healthcare infrastructure, and increasing incidences of respiratory infections in the region. With a diverse population and varying healthcare needs, countries like China, India, Japan, and South Korea are witnessing a surge in demand for advanced diagnostic solutions, particularly PCR-based assays and multiplex testing platforms. The region’s growing focus on early diagnosis and prompt treatment of respiratory infections, especially among vulnerable populations such as children, the elderly, and those with weakened immune systems, is fueling this demand. Additionally, governments and healthcare organizations are investing in improving healthcare access and diagnostic capabilities, contributing to market expansion.
The Human Metapneumovirus (hMPV) diagnostics market in China is growing, driven by an expanding healthcare infrastructure, increasing awareness of respiratory infections, and rising demand for advanced diagnostic solutions. With a large and diverse population, China faces a growing burden of respiratory diseases, particularly during seasonal outbreaks, leading to heightened demand for accurate and efficient diagnostics.
Japan Human Metapneumovirus (hMPV) diagnostics market is experiencing growth driven by the country’s advanced healthcare infrastructure, high healthcare standards, and strong demand for accurate diagnostic solutions.
The Human Metapneumovirus (hMPV) diagnostics market in India is experiencing growth driven by increasing awareness of respiratory illnesses and a growing demand for advanced diagnostic solutions. The rising prevalence of respiratory infections, particularly during seasonal outbreaks, has highlighted the need for accurate and rapid diagnostic tools in hospitals, clinics, and diagnostic laboratories. India’s improving healthcare infrastructure and government initiatives to enhance diagnostic capabilities are supporting the adoption of advanced technologies such as PCR-based assays and multiplex testing.
Latin America Human Metapneumovirus Diagnostics Market Trends
The Latin America Human Metapneumovirus (hMPV) diagnostics market is experiencing significant growth, driven by an increasing focus on improving healthcare infrastructure and growing awareness of respiratory illnesses. Countries such as Brazil, Mexico, and Argentina are leading the adoption of advanced diagnostic solutions, including PCR-based assays, to address the rising prevalence of respiratory infections.
The Human Metapneumovirus (hMPV) diagnostics market in Brazil is experiencing steady growth, driven by the increasing prevalence of respiratory infections and the country’s efforts to enhance healthcare infrastructure.
Middle East and Africa Human Metapneumovirus Diagnostics Market Trends
The Middle East and Africa Human Metapneumovirus (hMPV) diagnostics market is expanding driven by an increasing focus on improving healthcare infrastructure and addressing the rising burden of respiratory infections. The prevalence of respiratory illnesses, particularly among children and immunocompromised populations, is driving the demand for accurate and accessible diagnostic solutions in the region.
The Human Metapneumovirus (hMPV) diagnostics market in Saudi Arabia is characterized by rapid innovation, growing competition, and a dynamic regulatory environment. The rising prevalence of respiratory infections, particularly during seasonal changes, has heightened the demand for accurate and rapid diagnostic tools in hospitals, clinics, and diagnostic laboratories.
Key Human Metapneumovirus Diagnostics Company Insights
Key companies operating in the Human Metapneumovirus (hMPV) diagnostics industry include leading players in the diagnostics and healthcare technology sectors, driving innovation and market growth. Companies such as Roche Diagnostics, Thermo Fisher Scientific, Abbott Laboratories, and Bio-Rad Laboratories are at the forefront, offering advanced PCR-based and multiplex diagnostic assays for accurate and efficient detection of hMPV. These companies invest heavily in research and development to introduce novel technologies and improve the sensitivity and specificity of their diagnostic tools.
Other notable players include Qiagen, which provides molecular testing solutions, and Hologic, Inc., known for its high-throughput diagnostic platforms. Emerging companies and regional players are also contributing to the market by addressing local needs and expanding access to diagnostic services. Collaborative efforts, mergers, and acquisitions are common strategies among these companies to strengthen their portfolios and market presence. Together, these key players are shaping the future of the hMPV diagnostics market through technological advancements and global reach.
Key Human Metapneumovirus Diagnostics Companies:
The following are the leading companies in the human metapneumovirus (hMPV) diagnostics market. These companies collectively hold the largest market share and dictate industry trends.
- QuidelOrtho Corporation
- ARUP Laboratories
- Diasorin
- R-Biopharm AG
- Seegene Inc.
- ZeptoMetrix
- Abbott
- Thermo Fisher Scientific Inc.
Human Metapneumovirus Diagnostics MarketReport Scope
|
Report Attribute |
Details |
|
Market size value in 2025 |
USD 591.36 million |
|
Revenue forecast in 2030 |
USD 971.30 million |
|
Growth rate |
CAGR of 10.43% from 2025 to 2030 |
|
Actual data |
2018 - 2024 |
|
Forecast period |
2025 - 2030 |
|
Quantitative units |
Revenue in USD million/billion, and CAGR from 2025 to 2030 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Technology, end-use, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
|
Country scope |
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Norway; Denmark; Sweden; China; Japan; India; Australia; Thailand; South Korea; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait |
|
Key companies profiled |
QuidelOrtho Corporation; ARUP Laboratories; Diasorin; R-Biopharm AG; Seegene Inc.; ZeptoMetrix; Abbott; Thermo Fisher Scientific Inc. |
|
Customization scope |
Free report customization (equivalent up to 8 analyst working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. Explore purchase options |
Global Human Metapneumovirus Diagnostics Market Report Segmentation
This report forecasts revenue growth at global, regional, & country levels and provides an analysis of industry trends in each of the subsegments from 2018 to 2030. For this study, Grand View Research, Inc. has segmented the global Human Metapneumovirus (hMPV) diagnostics market report based on the technology, end-use, and region:
-
Technology Outlook (Revenue, USD Million, 2018 - 2030)
-
PCR-based Diagnostics
-
Next-generation Sequencing (NGS)
-
Immunofluorescence Assays
-
Lateral Flow Assays
-
Microarray Technology
-
Other Advanced Technologies
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals and Clinics
-
Diagnostic and Reference Laboratories
-
Academic and Research Institutions
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Norway
-
Denmark
-
Sweden
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global human metapneumovirus diagnostics market size was estimated at USD 548.11 million in 2024 and is expected to reach USD 591.36 million in 2025.
b. The global human metapneumovirus diagnostics market is expected to grow at a compound annual growth rate of 10.43% from 2025 to 2030 to reach USD 971.30 million by 2030.
b. North America dominated the Human Metapneumovirus (hMPV) diagnostics market with a share of 41.18% in 2024. This is attributable to presence of advanced healthcare infrastructure, high prevalence of respiratory infections, and increased demand for rapid and accurate diagnostic solutions.
b. Some key players operating in the human metapneumovirus diagnostics market include QuidelOrtho Corporation, ARUP Laboratories, Diasorin, R-Biopharm AG, Seegene Inc., ZeptoMetrix, Abbott, Thermo Fisher Scientific Inc.
b. Key factors that are driving the market growth include the rising prevalence of respiratory infections globally and the increasing awareness of hMPV as a significant cause of these illnesses. hMPV is particularly prevalent among vulnerable populations, such as young children, the elderly, and immunocompromised individuals.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."




