
Healthcare Contract Research Organization Market Size, Share & Trends Analysis Report By Type (Drug Discovery, Pre-clinical, And Clinical), By Service, By Therapeutic Area, By Molecule, By Region, And Segment Forecasts, 2025 - 2030
- Report ID: 978-1-68038-688-2
- Number of Report Pages: 200
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2024
- Forecast Period: 2025 - 2030
- Industry: Healthcare
Market Size & Trends
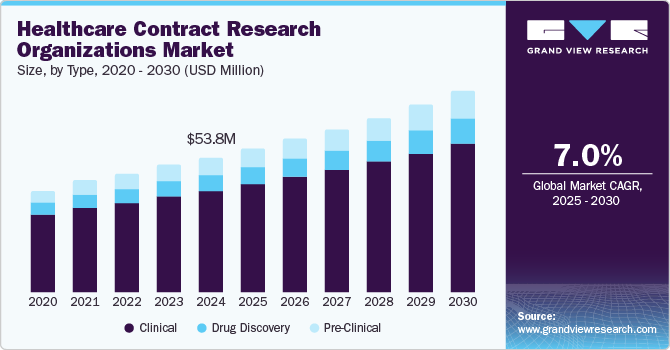
The global healthcare contract research organization market size was estimated at USD 53.84 billion in 2024 and is anticipated to grow at a CAGR of 7.0% from 2025 to 2030. Increasing investments by drug makers in R&D owing to patent expiration of blockbuster drugs is expected to be one of the major factors boosting the market. The diversified expertise of CROs compared to pharma companies with respect to performing clinical trials in a wide array of geographies and the development of drugs in specific therapeutic areas are some of the factors responsible for the growing demand for CROs in the pharmaceutical industry.
Increasing pressure on drug developers pertaining to clinical data management, regulatory environments, and stringent safety standards are expected to drive demand for contract research organizations within the healthcare sector. Healthcare and pharmaceutical companies are outsourcing the production of medicines and their clinical trials. With increasing clinical trial privatization, there is a surge in outsourcing to developing countries. Many healthcare Contract Research Organizations (CROs) are enhancing their global research network to provide better customer services. For instance, in February 2023, MMS, a CRO specializing in data, announced a collaboration with the Institute for Advanced Clinical Trials (I-ACT) to expedite the advancement of critical therapeutics such as medications, vaccines, and medical devices specifically personalized for pediatric use. As part of its commitment, MMS is sponsoring I-ACT's Spin Challenge, a unique initiative to generate funds to propel and hasten clinical trials focused on children's healthcare.
Pharmaceutical and medical device companies are increasingly outsourcing clinical research activities to CROs to stay competitive & flexible in the world of growing knowledge, gradually sophisticated technologies, & unstable economic environment. Companies preferring to outsource tasks include a wide range of activities from basic research to late-stage development, such as hit exploration & lead optimization, target validation, genetic engineering, assay development, and safety & efficacy tests in animal models & clinical trials involving humans. The race to launch molecules in the market in a feasible timeline and cost is expected to propel the need for CRO service providers for clinical research. Pharmaceutical companies face complexity in global regulations along with increased research costs. All these factors create the need for clinical research expertise in different portfolios, fueling the need for outsourcing in the market. Drug companies are increasingly outsourcing the production of medicines and clinical trials. With growing clinical trial privatization, there has been an increase in outsourcing of functions to developing countries such as India & China and the Latin American region.
The increase in the burden of non-communicable diseases (NCDs) and the rapidly growing aging population are factors contributing to the global health crisis. Rising life expectancy increases morbidity because people who live long enough develop more age-related diseases, dysfunction, and dementia. This is associated with high medical care costs and other challenges due to limited resources. The need to curb healthcare costs and proper management of chronic diseases are serious challenges that governments & other healthcare authorities face globally. Hence, digital technology is transforming the entire process of drug development. The advent of mobile & wearables, Artificial Intelligence (AI), cloud technology, and associated platforms now enables the collection of frequent, precise, & multidimensional data during trials. These advanced technologies have the potential to enable innovative trial designs that lead to easy recruitment & retention, improve patient experience, and establish novel endpoints in clinical studies.
Moreover, the evolving regulatory landscape and market dynamics drive merger and acquisition activities in the healthcare CRO sector. Companies aim to navigate regulatory obstacles and market uncertainties more effectively by leveraging the expertise & experience of their acquired targets. Strategic partnerships and acquisitions enable companies to mitigate risks, expedite regulatory approval processes, and ensure the successful commercialization of their products. For instance, in January 2023, Charles River Laboratories. acquired SAMDI Tech, Inc. that creates label-free high-throughput screening (HTS) solutions for drug discovery research. Similarly, in August 2023, Thermo Fisher Scientific Inc. acquired CorEvitas, LLC.
Market Concentration & Characteristics
The global healthcare contract research organization industry is characterized by a high degree of innovation, driven by advancements in clinical trial technologies, data analytics, and artificial intelligence. Companies are integrating decentralized trial models and precision medicine to enhance efficiency and patient outcomes. These innovations significantly reduce costs and timelines, addressing increasing complexity in drug development and regulatory demands.
Mergers and acquisitions dominate the healthcare CRO market as companies consolidate to enhance global reach, expertise, and service portfolios. Key players acquire specialized firms to address evolving client needs, streamline operations, and reduce competition. This trend bolsters market growth by fostering collaboration and innovation, enabling comprehensive services across preclinical, clinical, and post-market phases.

Stringent global regulatory requirements highly influence the healthcare CRO market. Compliance with Good Clinical Practice (GCP), FDA, and EMA standards drives operational precision and transparency. Regulatory complexities necessitate specialized expertise, pushing sponsors to outsource to CROs. Emerging regional regulations further emphasize the need for localized knowledge and adherence, shaping market operations globally.
Service expansion in the global healthcare CRO industry is characterized by increasing specialization in clinical trial services, including regulatory affairs and patient recruitment. CROs are expanding their geographic reach to cater to emerging markets, enhancing their global footprints. Healthcare CROs diversify their service portfolios to include end-to-end solutions across preclinical, clinical, and post-approval phases. Integrating real-world data, digital tools, and patient recruitment strategies enhances trial success rates. This expansion enables CROs to meet complex sponsor demands, improve market positioning, and capture a larger share of the pharmaceutical and biotechnology industries.
The key CROs in the market are undertaking various strategic initiatives to extend their operations and presence into new geographic regions.Regional expansion allows CROs to enter markets with specific needs or opportunities.
Type Insights
Clinical services accounted for the largest revenue share of 75.67% in 2024. With increasing research, biopharmaceutical companies must adhere to strict budgets and timelines, driving a preference for outsourcing to CROs or establishing clinical trial laboratories. The growing complexity of clinical trials, fueled by orphan diseases and cancer, necessitates new drug development approaches. Globalization further supports this trend as rising costs and patient recruitment challenges promote companies to regions like Central & Eastern Europe, Asia Pacific, Latin America, and the Middle East, offering cost savings and diverse disease profiles. Additionally, CROs collaborate with various organizations to expand geographic reach, enabling trials for rare diseases and addressing evolving customer demands effectively.
Preclinical studies is projected to witness a rapid growth of 8.61% during the forecast period. An increase in the number of preclinical trials globally and an increasing need to curb R&D expenses are expected to contribute to a growing demand for quality preclinical CRO services, thereby contributing to market growth. The presence of numerous CROs offering preclinical services is contributing to market growth. Players are adopting various strategic initiatives to remain competitive & gain market share. For instance, in February 2024, Charles River Laboratories International, Inc. announced an agreement with Wheeler Bio, Inc. This new partnership offered early-stage solutions to transition trials from preclinical to first-in-human clinical phases. Such actions by the market players are expected to promote segment growth.
Service Insights
Clinical monitoring dominated the market for healthcare contract research organizations and accounted for the largest revenue share of 19.91% in 2024. This can be attributed to an increasing number of clinical trials & the need to monitor those studies that are creating more demand for these services. Clinical research has been outsourced to CROs over the past decade due to various reasons, such as cost-effectiveness and technical expertise. The introduction of smart analytics along with real-time data acquisition devices is estimated to improve clinical monitoring data in the healthcare sector. Real-time data acquisition related to drug safety and toxicity enables early identification of trial errors and enables timely rectifications such as trial re-design or termination, thereby propelling segment growth. Moreover, several IT services and consulting companies are entering a field of clinical research through innovation across clinical monitoring platforms. For instance, in June 2023, ICON plc launched the most recent update of its Digital Platform. This advanced platform facilitates a smooth incorporation of ICON's site, patient, and sponsor services, ensuring a streamlined delivery of standardized data.
Regulatory/medical affairs is anticipated to witness the fastest growth rate of 10.62% in the healthcare CRO market over the forecast period. Outsourcing for regulatory affairs is expanding rapidly due to an increase in R&D activities, clinical trial applications, product registration, and drug pipelines. Increasing demand to obtain approval for new products, maintain compliance, and do more with less is projected to support segment growth. Also, CROs offering regulatory services are focusing on expansion strategies to boost their market presence. For instance, in September 2022, PharmaLex GMBH, a leading provider of regulatory services worldwide, announced the opening of a new office in China and Beijing, providing their clients access to a team of pharmaceutical and biopharmaceutical products-related regulatory experts in the region.
Therapeutic Area Insights
Oncology held the highest market share in 2024. The market for the segment is likely to expand with an increasing number of cancer cases across the globe. For instance, the Cancer Atlas mentioned that there will be 29 million cancer cases by 2040 globally. Furthermore, recent trends have shown a vast migration in pharmaceutical/medical device products with significant advancements in cancer treatment. In addition, the pharmaceutical sector has experienced significant growth due to the rising demand for oncology drugs & therapies, fueled by innovative targeted treatments, immunotherapies, and personalized medicine approaches. Increasing pharmaceutical R&D investments, patent expirations, and demand for oncology drugs & biologic innovations drive the oncology CRO market.
The CNS disorders segment is anticipated to grow at the fastest CAGR over the forecast period. CNS disorders account for about 8.0% of the global health burden, affecting many population groups. About 50 million Americans are affected by neurological disorders, and the numbers are similar across the globe. The incidence of neurological disorders is anticipated to increase with the growing elderly population. According to the WHO, over 1.4 billion people were aged 60 and above in 2020, which is expected to reach 2.1 billion by 2050. The increase in the aging population is one of the major reasons for the growing incidence of neurological disorders, such as Alzheimer’s, stroke, Parkinson’s, and others.
Molecule Insights
Pharmaceuticals dominated the healthcare Contract Research Organizations (CRO) market in 2024. Segment growth can be attributed to the increasing number of promising small molecules in the expanding global therapeutic market. In addition, the use of specialty medicines in the pharmaceutical industry is increasing globally, where small molecules such as APIs and finished drugs contribute significantly to sales. Thus, there is a rising demand for outsourcing services to CROs. These outsourcing services help reduce the expenditure of end users (customers) by enabling cost-effective drug production. They also aid in saving time, which can be used in the operations & management of a manufacturing & research facility. For instance, in 2022, as reported by the U.S. FDA, small molecules held around 59% of new drug approvals, representing 22 of the 37 new drugs approved in 2022.
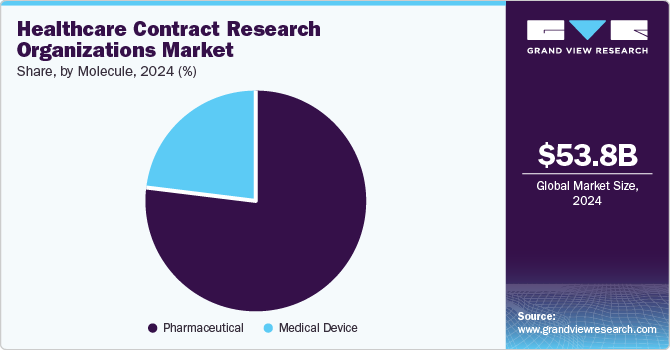
The biologics segment is expected to grow significantly during the forecast period. The segment growth can be attributed to increasing demand for advanced technologies for biological manufacturing, rising outsourcing of R&D activities by pharma and biopharma companies, increasing M&A activities, and a growing favorable regulatory environment in developing countries. For instance, in March 2023, Samsung Biologics invested USD 1.5 billion to set up a new plant in South Korea. The investment is expected to drive market demand.
The medical device segment is estimated to witness a considerable CAGR during the forecast period. The segment's expansion is expected to be driven by the range of medical device materials & applications, product design complexity, the increasing number of small to medium-sized medical device manufacturers, and strict approval norms. In addition, favorable government support has accelerated the demand for CROs around the globe. CROs provide a skilled medical device team with therapeutic knowledge and improved insights to support a smart strategy to get items to patients more quickly. Such factors are anticipated to promote the demand for CROs for research on medical devices.
Regional Insights
North America dominated the healthcare Contract Research Organizations market, accounting for a revenue share of 45.50% in 2024. This is due to the region's highest number of trials undertaken and outsourced. In addition, growing government support for R&D activities through grants and funds to research institutes and companies has driven this regional market. The rise of biologics and related biosimilars has been a major factor in the mergers & acquisitions of Contract Development & Manufacturing Organizations (CDMOs) and CROs. Developing biological and biosimilar drugs requires significantly more time & money than developing small molecules and generic drugs. Hence, smaller pharmaceutical clients have mainly outsourced to partners. Statistics show that while large drug sponsors outsource 40% of API production, smaller commercial biopharma companies currently outsource 73% of that process. To meet the growing demand for smaller pharmaceutical sponsors, middle-market independent CROs are collaborating with capital and strategic partners to make the required investments in talent & equipment.
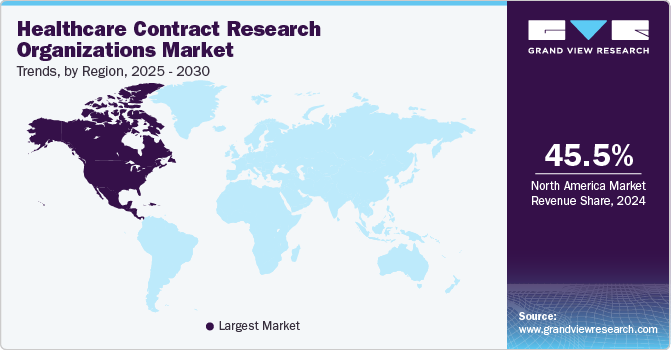
U.S. Healthcare Contract Research Organizations Market Trends
The healthcare Contract Research Organizations industry in the U.S. held the largest share in North America in 2024, driven by rising demand for outsourcing clinical trials and research activities. Increasing complexity in drug development, including precision medicine and biologics, prompts biopharmaceutical and pharmaceutical companies to collaborate with CROs for specialized expertise and infrastructure. Technological advancements, such as decentralized trials, AI-driven data analysis, and patient recruitment tools, are transforming the market landscape. Additionally, stringent FDA regulations and the need for compliance with Good Clinical Practice (GCP) guidelines encourage companies to partner with CROs for streamlined operations. The market is witnessing consolidation through mergers and acquisitions, enabling CROs to expand their service portfolios and geographic reach.
Europe Healthcare Contract Research Organizations Market Trends
The healthcare Contract Research Organizations market in Europe is expected to grow at a significant CAGR during the forecast period, owing to increasing clinical trial activity, the rise of precision medicine, and advancements in drug development. The region benefits from a highly skilled workforce, stringent regulatory frameworks, and a well-established pharmaceutical industry. Key trends include adopting decentralized clinical trials and digital technologies like artificial intelligence and blockchain, enhancing efficiency and patient engagement. The demand for specialized services, such as oncology and rare disease trials, is also rising, reflecting the growing complexity of research.
The UK healthcare Contract Research Organizations market held the largest share in Europe in 2024. The growth can be attributed to increasing clinical trials, rising demand for outsourcing services, and advancements in biotechnology. Key trends include adopting decentralized clinical trials, integrating AI and big data for analytics, and focusing on rare disease research. Stringent regulatory frameworks and Brexit-related adjustments also shape the market, with CROs expanding service portfolios and geographic reach to address evolving client needs and ensure compliance.
The healthcare Contract Research Organizations market in Germany is anticipated to grow at a significant CAGR over the forecast period. Growth in the country can be attributed to businesses' increased spending on R&D, rising healthcare awareness, and technological advancements. The country's emphasis on precision medicine and advanced therapeutic development drives innovation in trial methodologies. Increased collaboration between CROs and academic institutions accelerates research capabilities.
Asia Pacific Healthcare Contract Research Organizations Market Trends
The healthcare Contract Research Organizations market in Asia Pacific is expected to grow at the fastest CAGR over the forecast period. The growing patient pool and the outsourcing trend in the biopharmaceutical industry, particularly during the clinical stages of R&D, contribute to the growing number of clinical studies in the region. India has minimal regulations on the import & export of clinical supplies, making the process faster. However, China has a large patient pool with a few regulatory adjustments, which is expected to contribute to its largest market share. The ease of access to a treatment-naive patient pool in these countries drives advanced outsourcing of clinical trials and related activities.
Japan healthcare Contract Research Organizations market held the largest share in Asia Pacific in 2024. The market growth is owing to the expansion of the pharmaceutical sector, rising healthcare spending, and growing awareness and initiatives from the government and institutions.
The healthcare Contract Research Organizations market in India is expected to grow at the fastest CAGR over the forecast period due to its large patient population, cost-effectiveness, and growing expertise in clinical trials. The country is becoming a key destination for Phase II and III trials, particularly in oncology, cardiovascular, and infectious diseases. Increased investment in healthcare infrastructure, government support for research, and a favorable regulatory environment further boost CRO growth.
China healthcare Contract Research Organizations industry is anticipated to grow significantly over the forecast period. Growth in the country can be attributed to the strong government support for medical research, expanding biotechnology, and increasing demand for innovative therapies. The country is becoming a leading hub for early-phase trials, especially in oncology, neurology, and rare diseases. Local CROs leverage China’s vast patient pool, digital health technologies, and favorable regulatory policies.
Latin America Healthcare Contract Research Organizations Market Trends
The healthcare Contract Research Organizations market in Latin America is anticipated to grow at a substantial CAGR over the forecast period, owing to the increasing demand for clinical trials in diverse therapeutic areas, including oncology and infectious diseases. The region's diverse patient populations, lower trial costs, and emerging healthcare infrastructure attract global pharmaceutical companies. Additionally, regulatory reforms and collaborations between local CROs and international partners enhance research capabilities. The rise of digital technologies and data analytics further strengthens the region's position in global clinical research.
Brazil healthcare Contract Research Organizations market is anticipated to grow at a significant CAGR over the forecast period. Its large and diverse patient population provides unique opportunities for clinical trials, particularly in oncology, cardiovascular, and infectious diseases. The country's improving regulatory environment, along with a growing focus on research and development, enhances its attractiveness for global pharmaceutical companies.
MEA Healthcare Contract Research Organizations Market Trends
The healthcare Contract Research Organizations market in MEA is expected to grow at a substantial CAGR over the forecast period, driven by increasing demand for clinical trials in emerging therapeutic areas like oncology, diabetes, and cardiovascular diseases. Government investments in healthcare infrastructure and favorable regulatory frameworks boost the region’s appeal. The expanding healthcare sector, coupled with a rising number of multinational CROs, fosters regional collaborations, enhancing clinical trial execution, patient recruitment, and market penetration across MEA.
South Africa healthcare Contract Research Organizations industry is anticipated to grow at the fastest CAGR over the forecast period. As the country becomes a key destination for clinical trials in sub-Saharan Africa. The country's well-established infrastructure, diverse patient population, and competitive costs make it attractive for global pharmaceutical companies. Additionally, South Africa's strong regulatory framework, skilled workforce, and increasing focus on research in infectious diseases, oncology, and chronic conditions drive CRO demand, enhancing its position in the global healthcare contract research organizations industry landscape.
Key Healthcare Contract Research Organizations Company Insights
The prominent market players operating across the global market focus on implementing numerous strategic initiatives such as acquisitions, mergers, service launches, partnerships, expansions, and collaborations, among others, to broaden the geographical reach and gain a competitive edge in the overall market. For instance, in February 2024, Charles River Laboratories entered into an agreement with Pluristyx Inc. This provides C River access to research tools to support development of new therapeutics. Such collaborations broadened the service offerings of the company in the significant market.
Key Healthcare Contract Research Organizations Companies:
The following are the leading compfreelance platformsies in the healthcare contract research organizations market. These compfreelance platformsies collectively hold the largest market share freelance platformsd dictate industry trends.
- ICON Plc
- Charles River Laboratories
- Syneos Health
- IQVIA Inc.
- GVK Biosciences Private Limited (Aragen)
- LabCorp
- Parexel International Corporation
- Thermo Fisher Scientific
- CTI Clinical Trial & Consulting
- PSI
- Medpace
- Ergomed
- WuXi AppTec
- Worldwide Clinical Trials
- Medidata Solutions, Inc
- Pharmaron GMBH
- SGS SA
- KCR S.A.
- Advanced Clinical Research Services, LLC
- Pharm-Olam, LLC (Allucent)
View a comprehensive list of companies in the Healthcare Contract Research Organizations Market
Recent Developments
-
In March 2024, Thermo Fisher Scientific (PPD) Launched CorEvitas syndicated clinical registry in Generalized Pustular Psoriasis (GPP).
-
In January 2024, Parexel International Corporation and the Japanese Foundation for Cancer Research (JFCR) formed a strategic alliance to enhance access to oncology clinical trials in Japan.
-
In September 2023, ICON Plc., announced the release of its next-generation clinical trial tokenization solution. This solution provides insights into drug safety and efficacy throughout the product development lifecycle.
Health Contract Research Organizations Market Report Scope
|
Report Attribute |
Details |
|
Market size value in 2025 |
USD 57.42 billion |
|
Revenue forecast in 2030 |
USD 80.61 billion |
|
Growth rate |
CAGR of 7.0% from 2025 to 2030 |
|
Actual data |
2018 - 2024 |
|
Forecast period |
2025 - 2030 |
|
Quantitative units |
Revenue in USD billion and CAGR from 2025 to 2030 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Type, service, therapeutic area, molecule, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
|
Country scope |
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Switzerland; Belgium; Netherlands; Austria; Denmark; Sweden; Norway; India; China; Japan; South Korea; Taiwan; Australia; Indonesia; Malaysia; Singapore; Thailand; Brazil; Argentina; Colombia; Chile; Egypt; Saudi Arabia; UAE; Kuwait; Israel; South Africa |
|
Key companies profiled |
ICON Plc; Charles River Laboratories; Syneos Health; IQVIA Inc.; GVK Biosciences Private Limited (Aragen); LabCorp; Parexel International Corporation; Thermo Fisher Scientific; CTI Clinical Trial & Consulting; PSI; Medpace; Ergomed; WuXi AppTec; Worldwide Clinical Trials; Medidata Solutions, Inc; Pharmaron GMBH; SGS SA; KCR S.A.; Advanced Clinical Research Services, LLC; Pharm-Olam, LLC (Allucent) |
|
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. Explore purchase options |
Global Healthcare Contract Research Organizations Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 - 2030. For this study, Grand View Research has segmented the global healthcare Contract Research Organizations market report based on type, service, therapeutic area, molecule, and region:
-
Type Outlook (Revenue, USD Billion, 2018 - 2030)
-
Drug Discovery
-
Target Validation
-
Lead Identification
-
Lead Optimization
-
-
Pre-Clinical
-
Clinical
-
Phase I Trial Services
-
Phase II Trial Services
-
Phase III Trial Services
-
Phase IV Trial Services
-
-
-
Service Outlook (Revenue, USD Billion, 2018 - 2030)
-
Project Management/Clinical Supply Management
-
Data Management
-
Regulatory/Medical Affairs
-
Medical Writing
-
Clinical Monitoring
-
Quality Management/ Assurance
-
Bio-statistics
-
Investigator Payments
-
Laboratory
-
Sterility Testing
-
Container/Closure Testing
-
Extractables and Leachable Testing
-
Environmental Monitoring (Including Microbiology Testing)
-
Disinfectant Efficacy Studies
-
Others
-
-
Patient And Site Recruitment
-
Technology
-
Others
-
-
Therapeutic Area Outlook (Revenue, USD Billion, 2018 - 2030)
-
Oncology
-
CNS Disorders
-
Infectious Diseases
-
Immunological Disorders
-
Cardiovascular Diseases
-
Respiratory Diseases
-
Diabetes
-
Ophthalmology
-
Pain Management
-
Others
-
-
Molecule Outlook (Revenue, USD Billion, 2018 - 2030)
-
Pharmaceutical
-
Small Molecules
-
Biologics
-
-
Medical Device
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
Switzerland
-
Belgium
-
Netherlands
-
Austria
-
-
Asia Pacific
-
Japan
-
China
-
India
-
South Korea
-
Australia
-
Thailand
-
Taiwan
-
Indonesia
-
Malaysia
-
Singapore
-
-
Latin America
-
Brazil
-
Argentina
-
Colombia
-
Chile
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
Egypt
-
Israel
-
-
Frequently Asked Questions About This Report
b. The global healthcare contract research organization market size was estimated at USD 53.84 billion in 2024 and is expected to reach USD 57.42 billion in 2025.
b. The global healthcare contract research organization market is expected to grow at a compound annual growth rate of 7.02% from 2025 to 2030 to reach USD 80.61 billion in 2030.
b. Based on type, the clinical segment dominated the healthcare CRO market and accounted for the largest revenue share of 75.67% in 2024, driven by advancements in personalized medicine, AI integration, increased outsourcing, regulatory changes, and expanding clinical trials in emerging markets.
b. Some key players operating in the healthcare CRO market include ICON Plc, Charles River Laboratories, Syneos Health, IQVIA Inc., GVK Biosciences Private Limited (Aragen), LabCorp, Parexel International Corporation, Thermo Fisher Scientific Inc., CTI Clinical Trial & Consulting, PSI, Medpace, Ergomed, WuXi AppTec, Worldwide Clinical Trials, Medidata Solutions, Inc, Pharmaron GMBH, SGS SA, KCR S.A., Advanced Clinical Research Services, LLC, Pharm-Olam LLC (Allucent)
b. Key factors that are driving the healthcare contract research organization market growth include increasing investment in R&D programs, preference for outsourcing activities due to time and cost constraints, and patent expiration in the healthcare sector.
Table of Contents
Chapter 1. Methodology and Scope
1.1. Market Segmentation & Scope
1.1.1. Regional Scope
1.1.2. Estimates and Forecast Timeline
1.2. Market Definitions
1.3. Research Methodology
1.3.1. Information Procurement
1.3.2. Purchased Database
1.3.3. GVR’s Internal Database
1.3.4. Secondary Sources
1.3.5. Primary Research
1.4. Information or Data Analysis
1.4.1. Data Analysis Models
1.5. Market Formulation & Validation
1.5.1. Region Wise Market: Base Estimates
1.5.2. Global Market: CAGR Calculation
1.6. Model Details
1.6.1. Commodity Flow Analysis (Model 1)
1.6.2. Value-Chain-Based Sizing & Forecasting (Model 2)
1.6.3. QFD Model Sizing & Forecasting (Model 3)
1.6.4. Bottom-Up Approach (Model 4)
1.7. List of Secondary Sources
1.8. List of Abbreviations
1.9. Objectives
Chapter 2. Executive Summary
2.1. Market Outlook
2.2. Segment Snapshot
2.3. Competitive Landscape Snapshot
Chapter 3. Healthcare Contract Research Organizations (CRO) Market Variables, Trends & Scope
3.1. Market Lineage Outlook
3.1.1. Parent Market Outlook
3.1.2. Ancillary Market Outlook
3.2. Market Dynamics
3.2.1. Market Driver Analysis
3.2.1.1. Increasing Rate of Clinical Research To Boost Demand For Outsourcing Services
3.2.1.2. Increasing Adoption Of Advanced Technologies
3.2.1.3. Increasing Mergers and Collaborations
3.2.1.4. Increasing Demand For Outsourcing Services Across The Developing Economies
3.2.1.5. Patent Cliff
3.2.2. Market Restraint Analysis
3.2.2.1. Quality issues of CRO services
3.2.2.2. Intellectual property rights issues
3.3. Technological Advancements
3.3.1. Integration of AI and digital technologies
3.4. R&D Investment Perspective, by Sponsors
3.5. Clinical Trials Volume Analysis, 2024
3.5.1. Total Number of Clinical Trials, by Region (2024)
3.5.2. Total Number of Clinical Trials, by Phase (2024)
3.5.3. Total Number of Clinical Trials, by Study Design (2024)
3.5.4. Total Number of Clinical Trials, by Key Therapeutic Area (2024)
3.6. Market Analysis Tools
3.6.1. Porter’s Five Forces Analysis
3.6.2. PESTEL by SWOT Analysis
3.6.3. COVID-19 Impact Analysis
Chapter 4. Healthcare Contract Research Organizations (CRO) Market: Type Estimates & Trend Analysis
4.1. Segment Dashboard
4.2. Global Healthcare Contract Research Organizations (CRO) Market Movement Analysis
4.3. Global Healthcare Contract Research Organizations (CRO) Market Size & Trend Analysis, by Type, 2018 - 2030 (USD Million)
4.4. Drug Discovery
4.4.1. Drug Discovery Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
4.4.2. Target Validation
4.4.2.1. Target Validation Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
4.4.3. Lead Identification
4.4.3.1. Lead Identification Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
4.4.4. Lead Optimization
4.4.4.1. Lead Optimization Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
4.5. Pre-clinical
4.5.1. Pre-clinical Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
4.6. Clinical
4.6.1. Clinical Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
4.6.2. Phase I Trial Services
4.6.2.1. Phase I Trial Services Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
4.6.3. Phase II Trial Services
4.6.3.1. Phase II Trial Services Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
4.6.4. Phase III Trial Services
4.6.4.1. Phase III Trial Services Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
4.6.5. Phase IV Trial Services
4.6.5.1. Phase IV Trial Services Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
Chapter 5. Healthcare Contract Research Organizations (CRO) Market: Service Estimates & Trend Analysis
5.1. Segment Dashboard
5.2. Global Healthcare Contract Research Organizations (CRO) Market Movement Analysis
5.3. Global Healthcare Contract Research Organizations (CRO) Market Size & Trend Analysis, by Service, 2018 - 2030 (USD Million)
5.4. Project Management/Clinical Supply Management
5.4.1. Project Management/Clinical Supply Management Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
5.5. Data Management
5.5.1. Data Management Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
5.6. Regulatory/Medical Affairs
5.6.1. Regulatory/Medical Affairs Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
5.7. Medical Writing
5.7.1. Medical Writing Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
5.8. Clinical Monitoring
5.8.1. Clinical Monitoring Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
5.9. Quality Management/Assurance
5.9.1. Quality Management/Assurance Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
5.10. Bio-statistics
5.10.1. Bio-statistics Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
5.11. Investigator Payments
5.11.1. Investigator Payments Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
5.12. Laboratory
5.12.1. Laboratory Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
5.12.2. Sterility Testing
5.12.2.1. Sterility Testing Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
5.12.3. Container/Closure Testing
5.12.3.1. Container/Closure Testing Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
5.12.4. Extractables and Leachable Testing
5.12.4.1. Extractables and Leachable Testing Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
5.12.5. Environmental Monitoring
5.12.5.1. Environmental Monitoring Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
5.12.6. Disinfectant Efficacy Studies
5.12.6.1. Disinfectant Efficacy Studies Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
5.12.7. Others
5.12.7.1. Others Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
5.13. Patient and Site Recruitment
5.13.1. Patient and Site Recruitment Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
5.14. Technology
5.14.1. Technology Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
5.15. Others
5.15.1. Others Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
Chapter 6. Healthcare Contract Research Organizations (CRO) Market: Therapeutic Area Estimates & Trend Analysis
6.1. Segment Dashboard
6.2. Global Healthcare Contract Research Organizations (CRO) Market Movement Analysis
6.3. Global Healthcare Contract Research Organizations (CRO) Market Size & Trend Analysis, by Therapeutic Area, 2018 - 2030 (USD Million)
6.4. Oncology
6.4.1. Oncology Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5. CNS Disorders
6.5.1. CNS Disorders Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.6. Infectious Diseases
6.6.1. Infectious Diseases Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.7. Immunological Disorders
6.7.1. Immunological Disorders Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.8. Cardiovascular Diseases
6.8.1. Cardiovascular Diseases Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.9. Respiratory Diseases
6.9.1. Respiratory Diseases Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.10. Diabetes
6.10.1. Diabetes Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.11. Ophthalmology
6.11.1. Ophthalmology Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.12. Pain Management
6.12.1. Pain Management Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.13. Others
6.13.1. Others Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Chapter 7. Healthcare Contract Research Organizations (CRO) Market: Molecule Estimates & Trend Analysis
7.1. Segment Dashboard
7.2. Global Healthcare Contract Research Organizations (CRO) Market Movement Analysis
7.3. Global Healthcare Contract Research Organizations (CRO) Market Size & Trend Analysis, by Molecule, 2018 - 2030 (USD Million)
7.4. Pharmaceuticals
7.4.1. Pharmaceuticals Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
7.4.2. Small Molecules
7.4.2.1. Small Molecules Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
7.4.3. Biologics
7.4.3.1. Biologics Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
7.5. Medical Device
7.5.1. Medical Device Healthcare Contract Research Organization Market, 2018 - 2030 (USD Million)
Chapter 8. Healthcare Contract Research Organizations (CRO) Market: Regional Estimates & Trend Analysis
8.1. Regional Market Dashboard
8.2. Global Regional Market Snapshot
8.3. Market Size & Forecasts Trend Analysis, 2018 - 2030:
8.4. North America
8.4.1. North America Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.4.2. U.S.
8.4.2.1. Key Country Dynamics
8.4.2.2. Competitive Scenario
8.4.2.3. Regulatory Framework
8.4.2.4. U.S. Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.4.3. Canada
8.4.3.1. Key Country Dynamics
8.4.3.2. Competitive Scenario
8.4.3.3. Regulatory Framework
8.4.3.4. Canada Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.4.4. Mexico
8.4.4.1. Key Country Dynamics
8.4.4.2. Competitive Scenario
8.4.4.3. Regulatory Framework
8.4.4.4. Mexico Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5. Europe
8.5.1. Europe Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.2. UK
8.5.2.1. Key Country Dynamics
8.5.2.2. Competitive Scenario
8.5.2.3. Regulatory Framework
8.5.2.4. UK Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.3. Germany
8.5.3.1. Key Country Dynamics
8.5.3.2. Competitive Scenario
8.5.3.3. Regulatory Framework
8.5.3.4. Germany Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.4. France
8.5.4.1. Key Country Dynamics
8.5.4.2. Competitive Scenario
8.5.4.3. Regulatory Framework
8.5.4.4. France Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.5. Italy
8.5.5.1. Key Country Dynamics
8.5.5.2. Competitive Scenario
8.5.5.3. Regulatory Framework
8.5.5.4. Italy Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.6. Spain
8.5.6.1. Key Country Dynamics
8.5.6.2. Competitive Scenario
8.5.6.3. Regulatory Framework
8.5.6.4. Spain Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.7. Denmark
8.5.7.1. Key Country Dynamics
8.5.7.2. Competitive Scenario
8.5.7.3. Regulatory Framework
8.5.7.4. Denmark Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.8. Sweden
8.5.8.1. Key Country Dynamics
8.5.8.2. Competitive Scenario
8.5.8.3. Regulatory Framework
8.5.8.4. Sweden Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.9. Norway
8.5.9.1. Key Country Dynamics
8.5.9.2. Competitive Scenario
8.5.9.3. Regulatory Framework
8.5.9.4. Norway Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.10. Switzerland
8.5.10.1. Key Country Dynamics
8.5.10.2. Competitive Scenario
8.5.10.3. Regulatory Framework
8.5.10.4. Switzerland Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.11. Belgium
8.5.11.1. Key Country Dynamics
8.5.11.2. Competitive Scenario
8.5.11.3. Regulatory Framework
8.5.11.4. Belgium Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.12. Netherlands
8.5.12.1. Key Country Dynamics
8.5.12.2. Competitive Scenario
8.5.12.3. Regulatory Framework
8.5.12.4. Netherlands Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.13. Austria
8.5.13.1. Key Country Dynamics
8.5.13.2. Competitive Scenario
8.5.13.3. Regulatory Framework
8.5.13.4. Austria Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6. Asia Pacific
8.6.1. Asia Pacific Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.2. China
8.6.2.1. Key Country Dynamics
8.6.2.2. Competitive Scenario
8.6.2.3. Regulatory Framework
8.6.2.4. China Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.3. Japan
8.6.3.1. Key Country Dynamics
8.6.3.2. Competitive Scenario
8.6.3.3. Regulatory Framework
8.6.3.4. Japan Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.4. India
8.6.4.1. Key Country Dynamics
8.6.4.2. Competitive Scenario
8.6.4.3. Regulatory Framework
8.6.4.4. India Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.5. Australia
8.6.5.1. Key Country Dynamics
8.6.5.2. Competitive Scenario
8.6.5.3. Regulatory Framework
8.6.5.4. Australia Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.6. South Korea
8.6.6.1. Key Country Dynamics
8.6.6.2. Competitive Scenario
8.6.6.3. Regulatory Framework
8.6.6.4. South Korea Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.7. Thailand
8.6.7.1. Key Country Dynamics
8.6.7.2. Competitive Scenario
8.6.7.3. Regulatory Framework
8.6.7.4. Thailand Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.8. Taiwan
8.6.8.1. Key Country Dynamics
8.6.8.2. Competitive Scenario
8.6.8.3. Regulatory Framework
8.6.8.4. Taiwan Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.9. Indonesia
8.6.9.1. Key Country Dynamics
8.6.9.2. Competitive Scenario
8.6.9.3. Regulatory Framework
8.6.9.4. Indonesia Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.10. Malaysia
8.6.10.1. Key Country Dynamics
8.6.10.2. Competitive Scenario
8.6.10.3. Regulatory Framework
8.6.10.4. Malaysia Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.11. Singapore
8.6.11.1. Key Country Dynamics
8.6.11.2. Competitive Scenario
8.6.11.3. Regulatory Framework
8.6.11.4. Singapore Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.7. Latin America
8.7.1. Latin America Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.7.2. Brazil
8.7.2.1. Key Country Dynamics
8.7.2.2. Competitive Scenario
8.7.2.3. Regulatory Framework
8.7.2.4. Brazil Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.7.3. Argentina
8.7.3.1. Key Country Dynamics
8.7.3.2. Competitive Scenario
8.7.3.3. Regulatory Framework
8.7.3.4. Argentina Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.7.4. Colombia
8.7.4.1. Key Country Dynamics
8.7.4.2. Competitive Scenario
8.7.4.3. Regulatory Framework
8.7.4.4. Colombia Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.7.5. Chile
8.7.5.1. Key Country Dynamics
8.7.5.2. Competitive Scenario
8.7.5.3. Regulatory Framework
8.7.5.4. Chile Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.8. MEA
8.8.1. MEA Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.8.2. South Africa
8.8.2.1. Key Country Dynamics
8.8.2.2. Competitive Scenario
8.8.2.3. Regulatory Framework
8.8.2.4. South Africa Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.8.3. Saudi Arabia
8.8.3.1. Key Country Dynamics
8.8.3.2. Competitive Scenario
8.8.3.3. Regulatory Framework
8.8.3.4. Saudi Arabia Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.8.4. UAE
8.8.4.1. Key Country Dynamics
8.8.4.2. Competitive Scenario
8.8.4.3. Regulatory Framework
8.8.4.4. UAE Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.8.5. Kuwait
8.8.5.1. Key Country Dynamics
8.8.5.2. Competitive Scenario
8.8.5.3. Regulatory Framework
8.8.5.4. Kuwait Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.8.6. Egypt
8.8.6.1. Key Country Dynamics
8.8.6.2. Competitive Scenario
8.8.6.3. Regulatory Framework
8.8.6.4. Egypt Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.8.7. Israel
8.8.7.1. Key Country Dynamics
8.8.7.2. Competitive Scenario
8.8.7.3. Regulatory Framework
8.8.7.4. Israel Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Chapter 9. Competitive Landscape
9.1. Market Participant Categorization
9.1.1. Market Leaders
9.1.2. Emerging Players
9.2. Competitive Market Share/Assessment Analysis, 2024
9.3. Company Profiles
9.3.1. ICON Plc
9.3.1.1. Company Overview
9.3.1.2. Financial Performance
9.3.1.3. Service Benchmarking
9.3.1.4. Strategic Initiatives
9.3.2. Charles River Laboratories
9.3.2.1. Company Overview
9.3.2.2. Financial Performance
9.3.2.3. Service Benchmarking
9.3.2.4. Strategic Initiatives
9.3.3. Syneos Health
9.3.3.1. Company Overview
9.3.3.2. Financial Performance
9.3.3.3. Service Benchmarking
9.3.3.4. Strategic Initiatives
9.3.4. IQVIA Inc.
9.3.4.1. Company Overview
9.3.4.2. Financial Performance
9.3.4.3. Service Benchmarking
9.3.4.4. Strategic Initiatives
9.3.5. GVK Biosciences Private Limited (Aragen)
9.3.5.1. Company Overview
9.3.5.2. Financial Performance
9.3.5.3. Service Benchmarking
9.3.5.4. Strategic Initiatives
9.3.6. LabCorp
9.3.6.1. Company Overview
9.3.6.2. Financial Performance
9.3.6.3. Service Benchmarking
9.3.6.4. Strategic Initiatives
9.3.7. Parexel International Corporation
9.3.7.1. Company Overview
9.3.7.2. Financial Performance
9.3.7.3. Service Benchmarking
9.3.7.4. Strategic Initiatives
9.3.8. WuXi AppTec
9.3.8.1. Company Overview
9.3.8.2. Financial Performance
9.3.8.3. Service Benchmarking
9.3.8.4. Strategic Initiatives
9.3.9. Thermo Fisher Scientific Inc.
9.3.9.1. Company Overview
9.3.9.2. Financial Performance
9.3.9.3. Service Benchmarking
9.3.9.4. Strategic Initiatives
9.3.10. CTI Clinical Trial & Consulting
9.3.10.1. Company Overview
9.3.10.2. Financial Performance
9.3.10.3. Service Benchmarking
9.3.10.4. Strategic Initiatives
9.3.11. PSI
9.3.11.1. Company Overview
9.3.11.2. Financial Performance
9.3.11.3. Service Benchmarking
9.3.11.4. Strategic Initiatives
9.3.12. Medpace
9.3.12.1. Company Overview
9.3.12.2. Financial Performance
9.3.12.3. Service Benchmarking
9.3.12.4. Strategic Initiatives
9.3.13. Ergomed
9.3.13.1. Company Overview
9.3.13.2. Financial Performance
9.3.13.3. Service Benchmarking
9.3.13.4. Strategic Initiatives
9.3.14. Worldwide Clinical Trials
9.3.14.1. Company Overview
9.3.14.2. Financial Performance
9.3.14.3. Service Benchmarking
9.3.14.4. Strategic Initiatives
9.3.15. Medidata Solutions, Inc.
9.3.15.1. Company Overview
9.3.15.2. Financial Performance
9.3.15.3. Service Benchmarking
9.3.15.4. Strategic Initiatives
9.3.16. Pharmaron GMBH
9.3.16.1. Company Overview
9.3.16.2. Financial Performance
9.3.16.3. Service Benchmarking
9.3.16.4. Strategic Initiatives
9.3.17. SGS SA
9.3.17.1. Company Overview
9.3.17.2. Financial Performance
9.3.17.3. Service Benchmarking
9.3.17.4. Strategic Initiatives
9.3.18. KCR S.A.
9.3.18.1. Company Overview
9.3.18.2. Financial Performance
9.3.18.3. Service Benchmarking
9.3.18.4. Strategic Initiatives
9.3.19. Advanced Clinical Research Services, LLC.
9.3.19.1. Company Overview
9.3.19.2. Financial Performance
9.3.19.3. Service Benchmarking
9.3.19.4. Strategic Initiatives
9.3.20. Pharm-Olam, LLC (Allucent)
9.3.20.1. Company Overview
9.3.20.2. Financial Performance
9.3.20.3. Service Benchmarking
9.3.20.4. Strategic Initiatives
List of Tables
Table 1 List of Secondary Sources
Table 2 List of Abbreviations
Table 3 Global Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 4 Global Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 5 Global Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 6 Global Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 7 Global Nucleic Acid Therapeutics CDMO, by Region, 2018 - 2030 (USD Million)
Table 8 North America Nucleic Acid Therapeutics CDMO, by Country, 2018 - 2030 (USD Million)
Table 9 North America Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 10 North America Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 11 North America Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 12 North America Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 13 U.S. Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 14 U.S. Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 15 U.S. Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 16 U.S. Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 17 Canada Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 18 Canada Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 19 Canada Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 20 Canada Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 21 Mexico Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 22 Mexico Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 23 Mexico Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 24 Mexico Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 25 Europe Nucleic Acid Therapeutics CDMO, by Country, 2018 - 2030 (USD Million)
Table 26 Europe Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 27 Europe Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 28 Europe Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 29 Europe Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 30 Germany Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 31 Germany Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 32 Germany Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 33 Germany Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 34 UK Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 35 UK Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 36 UK Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 37 UK Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 38 France Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 39 France Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 40 France Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 41 France Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 42 Italy Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 43 Italy Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 44 Italy Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 45 Italy Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 46 Spain Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 47 Spain Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 48 Spain Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 49 Spain Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 50 Denmark Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 51 Denmark Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 52 Denmark Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 53 Denmark Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 54 Sweden Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 55 Sweden Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 56 Sweden Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 57 Sweden Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 58 Norway Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 59 Norway Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 60 Norway Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 61 Norway Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 62 Switzerland Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 63 Switzerland Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 64 Switzerland Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 65 Switzerland Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 66 Belgium Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 67 Belgium Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 68 Belgium Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 69 Belgium Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 70 Netherlands Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 71 Netherlands Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 72 Netherlands Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 73 Netherlands Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 74 Austria Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 75 Austria Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 76 Austria Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 77 Austria Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 78 Asia Pacific Nucleic Acid Therapeutics CDMO, by Country, 2018 - 2030 (USD Million)
Table 79 Asia Pacific Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 80 Asia Pacific Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 81 Asia Pacific Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 82 Asia Pacific Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 83 China Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 84 China Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 85 China Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 86 China Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 87 Japan Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 88 Japan Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 89 Japan Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 90 Japan Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 91 India Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 92 India Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 93 India Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 94 India Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 95 South Korea Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 96 South Korea Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 97 South Korea Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 98 South Korea Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 99 Australia Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 100 Australia Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 101 Australia Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 102 Australia Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 103 Thailand Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 104 Thailand Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 105 Thailand Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 106 Thailand Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 107 Taiwan Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 108 Taiwan Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 109 Taiwan Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 110 Taiwan Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 111 Indonesia Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 112 Indonesia Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 113 Indonesia Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 114 Indonesia Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 115 Malaysia Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 116 Malaysia Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 117 Malaysia Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 118 Malaysia Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 119 Singapore Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 120 Singapore Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 121 Singapore Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 122 Singapore Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 123 Latin America Nucleic Acid Therapeutics CDMO, by Country, 2018 - 2030 (USD Million)
Table 124 Latin America Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 125 Latin America Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 126 Latin America Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 127 Latin America Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 128 Brazil Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 129 Brazil Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 130 Brazil Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 131 Brazil Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 132 Argentina Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 133 Argentina Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 134 Argentina Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 135 Argentina Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 136 Colombia Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 137 Colombia Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 138 Colombia Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 139 Colombia Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 140 Chile Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 141 Chile Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 142 Chile Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 143 Chile Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 144 Middle East & Africa Nucleic Acid Therapeutics CDMO, by Country, 2018 - 2030 (USD Million)
Table 145 Middle East & Africa Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 146 Middle East & Africa Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 147 Middle East & Africa Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 148 Middle East & Africa Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 149 South Africa Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 150 South Africa Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 151 South Africa Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 152 South Africa Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 153 Saudi Arabia Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 154 Saudi Arabia Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 155 Saudi Arabia Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 156 Saudi Arabia Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 157 UAE Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 158 UAE Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 159 UAE Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 160 UAE Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 161 Kuwait Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 162 Kuwait Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 163 Kuwait Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 164 Kuwait Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 165 Egypt Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 166 Egypt Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 167 Egypt Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 168 Egypt Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
Table 169 Israel Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
Table 170 Israel Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
Table 171 Israel Nucleic Acid Therapeutics CDMO, by Therapeutic Area, 2018 - 2030 (USD Million)
Table 172 Israel Nucleic Acid Therapeutics CDMO, by Molecule, 2018 - 2030 (USD Million)
List of Figures
Fig. 1 Information Procurement
Fig. 2 Primary Research Pattern
Fig. 3 Market Research Approaches
Fig. 4 Value Chain-Based Sizing & Forecasting
Fig. 5 Market Formulation & Validation
Fig. 6 Healthcare Contract Research Organizations (CRO), Market Segmentation
Fig. 7 Market Driver Relevance Analysis (Current & Future Impact)
Fig. 8 Market Restraint Relevance Analysis (Current & Future Impact)
Fig. 9 SWOT Analysis, By Factor (Political & Legal, Economic and Technological)
Fig. 10 Porter’s Five Forces Analysis
Fig. 11 Regional Marketplace: Key Takeaways
Fig. 12 Global Healthcare Contract Research Organizations (CRO), for Drug Discovery, 2018 - 2030 (USD Million)
Fig. 13 Global Healthcare Contract Research Organizations (CRO), for Target Validation, 2018 - 2030 (USD Million)
Fig. 14 Global Healthcare Contract Research Organizations (CRO), for Lead Identification, 2018 - 2030 (USD Million)
Fig. 15 Global Healthcare Contract Research Organizations (CRO), for Lead Optimization, 2018 - 2030 (USD Million)
Fig. 16 Global Healthcare Contract Research Organizations (CRO), for Pre-Clinical, 2018 - 2030 (USD Million)
Fig. 17 Global Healthcare Contract Research Organizations (CRO), for Clinical, 2018 - 2030 (USD Million)
Fig. 18 Global Healthcare Contract Research Organizations (CRO), for Phase I Trials, 2018 - 2030 (USD Million)
Fig. 19 Global Healthcare Contract Research Organizations (CRO), for Phase II Trials, 2018 - 2030 (USD Million)
Fig. 20 Global Healthcare Contract Research Organizations (CRO), for Phase III Trials, 2018 - 2030 (USD Million)
Fig. 21 Global Healthcare Contract Research Organizations (CRO), for Phase IV Trials, 2018 - 2030 (USD Million)
Fig. 22 Global Healthcare Contract Research Organizations (CRO), for Project Management/Clinical Supply Management, 2018 - 2030 (USD Million)
Fig. 23 Global Healthcare Contract Research Organizations (CRO), for Data Management, 2018 - 2030 (USD Million)
Fig. 24 Global Healthcare Contract Research Organizations (CRO), for Regulatory/Medical Affairs, 2018 - 2030 (USD Million)
Fig. 25 Global Healthcare Contract Research Organizations (CRO), for Medical Writing, 2018 - 2030 (USD Million)
Fig. 26 Global Healthcare Contract Research Organizations (CRO), for Clinical Monitoring, 2018 - 2030 (USD Million)
Fig. 27 Global Healthcare Contract Research Organizations (CRO), for Quality Management/ Assurance, 2018 - 2030 (USD Million)
Fig. 28 Global Healthcare Contract Research Organizations (CRO), for Bio-statistics, 2018 - 2030 (USD Million)
Fig. 29 Global Healthcare Contract Research Organizations (CRO), for Investigator Payments , 2018 - 2030 (USD Million)
Fig. 30 Global Healthcare Contract Research Organizations (CRO), for Laboratory, 2018 - 2030 (USD Million)
Fig. 31 Global Healthcare Contract Research Organizations (CRO), for Sterility Testing, 2018 - 2030 (USD Million)
Fig. 32 Global Healthcare Contract Research Organizations (CRO), for Container/Closure Testing, 2018 - 2030 (USD Million)
Fig. 33 Global Healthcare Contract Research Organizations (CRO), for Extractables and Leachable Testing, 2018 - 2030 (USD Million)
Fig. 34 Global Healthcare Contract Research Organizations (CRO), for Environmental Monitoring (Including Microbiology Testing), 2018 - 2030 (USD Million)
Fig. 35 Global Healthcare Contract Research Organizations (CRO), for Disinfectant Efficacy Studies, 2018 - 2030 (USD Million)
Fig. 36 Global Healthcare Contract Research Organizations (CRO), for Others, 2018 - 2030 (USD Million)
Fig. 37 Global Healthcare Contract Research Organizations (CRO), for Patient and Site Recruitment , 2018 - 2030 (USD Million)
Fig. 38 Global Healthcare Contract Research Organizations (CRO), for Technology , 2018 - 2030 (USD Million)
Fig. 39 Global Healthcare Contract Research Organizations (CRO), for Others, 2018 - 2030 (USD Million)
Fig. 40 Global Healthcare Contract Research Organizations (CRO), for Oncology , 2018 - 2030 (USD Million)
Fig. 41 Global Healthcare Contract Research Organizations (CRO), for CNS Disorders , 2018 - 2030 (USD Million)
Fig. 42 Global Healthcare Contract Research Organizations (CRO), for Infectious Diseases , 2018 - 2030 (USD Million)
Fig. 43 Global Healthcare Contract Research Organizations (CRO), for Immunological Disorders , 2018 - 2030 (USD Million)
Fig. 44 Global Healthcare Contract Research Organizations (CRO), for Cardiovascular Diseases, 2018 - 2030 (USD Million)
Fig. 45 Global Healthcare Contract Research Organizations (CRO), for Respiratory Diseases , 2018 - 2030 (USD Million)
Fig. 46 Global Healthcare Contract Research Organizations (CRO), for Diabetes , 2018 - 2030 (USD Million)
Fig. 47 Global Healthcare Contract Research Organizations (CRO), for Ophthalmology , 2018 - 2030 (USD Million)
Fig. 48 Global Healthcare Contract Research Organizations (CRO), for Pain Management, 2018 - 2030 (USD Million)
Fig. 49 Global Healthcare Contract Research Organizations (CRO), for Others, 2018 - 2030 (USD Million)
Fig. 50 Global Healthcare Contract Research Organizations (CRO), for Pharmaceuticals, 2018 - 2030 (USD Million)
Fig. 51 Global Healthcare Contract Research Organizations (CRO), for Small Molecules, 2018 - 2030 (USD Million)
Fig. 52 Global Healthcare Contract Research Organizations (CRO), for Biologics, 2018 - 2030 (USD Million)
Fig. 53 Global Healthcare Contract Research Organizations (CRO), for Medical Device, 2018 - 2030 (USD Million)
Fig. 54 Regional Outlook, 2024 & 2030
Fig. 55 North America Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 56 US Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 57 Canada Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 58 Mexico Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 59 Europe Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 60 UK Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 61 Germany Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 62 France Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 63 Italy Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 64 Spain Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 65 Denmark Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 66 Sweden Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 67 Norway Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 68 Switzerland Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 69 Belgium Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 70 Netherlands Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 71 Austria Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 72 Asia Pacific Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 73 India Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 74 China Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 75 Japan Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 76 South Korea Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 77 Australia Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 78 Thailand Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 79 Taiwan Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 80 Indonesia Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 81 Malaysia Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 82 Singapore Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 83 Latin America Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 84 Brazil Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 85 Argentina Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 86 Colombia Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 87 Chile Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 88 MEA Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 89 South Africa Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 90 Saudi Arabia Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 91 UAE Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 92 Kuwait Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 93 Egypt Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Fig. 94 Israel Healthcare Contract Research Organizations (CRO) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Market Segmentation
- Healthcare Contract Research Organizations (CRO) Type Outlook (Revenue, USD Billion, 2018 - 2030)
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Healthcare Contract Research Organizations (CRO) Service Outlook (Revenue, USD Billion, 2018 - 2030)
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Healthcare Contract Research Organizations (CRO) Therapeutic Area Outlook (Revenue, USD Billion, 2018 - 2030)
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Healthcare Contract Research Organizations (CRO) Molecule Outlook (Revenue, USD Billion, 2018 - 2030)
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Pharmaceutical
- Healthcare Contract Research Organizations (CRO) Regional Outlook (Revenue, USD Million, 2018 - 2030)
- North America
- North America Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- North America Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- North America Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- North America Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- U.S.
- U.S. Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- U.S. Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- U.S. Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- U.S. Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- U.S. Healthcare Contract Research Organizations (CRO) Market, By Type
- Canada
- Canada Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Canada Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Canada Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Canada Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Canada Healthcare Contract Research Organizations (CRO) Market, By Type
- Mexico
- Mexico Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Mexico Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Mexico Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Mexico Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Mexico Healthcare Contract Research Organizations (CRO) Market, By Type
- North America Healthcare Contract Research Organizations (CRO) Market, By Type
- Europe
- Europe Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Europe Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Europe Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Europe Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- UK
- UK Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- UK Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- UK Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- UK Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- UK Healthcare Contract Research Organizations (CRO) Market, By Type
- Germany
- Germany Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Germany Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Germany Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Germany Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Germany Healthcare Contract Research Organizations (CRO) Market, By Type
- France
- France Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- France Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- France Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- France Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- France Healthcare Contract Research Organizations (CRO) Market, By Type
- Italy
- Italy Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Italy Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Italy Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Italy Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Italy Healthcare Contract Research Organizations (CRO) Market, By Type
- Spain
- Spain Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Spain Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Spain Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Spain Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Spain Healthcare Contract Research Organizations (CRO) Market, By Type
- Denmark
- Denmark Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Denmark Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Denmark Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Denmark Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Denmark Healthcare Contract Research Organizations (CRO) Market, By Type
- Sweden
- Sweden Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Sweden Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Sweden Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Sweden Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Sweden Healthcare Contract Research Organizations (CRO) Market, By Type
- Norway
- Norway Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Norway Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Norway Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Norway Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Norway Healthcare Contract Research Organizations (CRO) Market, By Type
- Switzerland
- Switzerland Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Switzerland Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Switzerland Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Switzerland Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Switzerland Healthcare Contract Research Organizations (CRO) Market, By Type
- Belgium
- Belgium Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Belgium Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Belgium Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Belgium Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Belgium Healthcare Contract Research Organizations (CRO) Market, By Type
- Netherlands
- Netherlands Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Netherlands Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Netherlands Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Netherlands Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Netherlands Healthcare Contract Research Organizations (CRO) Market, By Type
- Austria
- Austria Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Austria Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Austria Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Austria Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Austria Healthcare Contract Research Organizations (CRO) Market, By Type
- Europe Healthcare Contract Research Organizations (CRO) Market, By Type
- Asia Pacific
- Asia Pacific Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Asia Pacific Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Asia Pacific Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Asia Pacific Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Japan
- Japan Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Japan Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Japan Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Japan Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Japan Healthcare Contract Research Organizations (CRO) Market, By Type
- China
- China Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- China Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- China Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- China Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- China Healthcare Contract Research Organizations (CRO) Market, By Type
- India
- India Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- India Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- India Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- India Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- India Healthcare Contract Research Organizations (CRO) Market, By Type
- South Korea
- South Korea Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- South Korea Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- South Korea Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- South Korea Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- South Korea Healthcare Contract Research Organizations (CRO) Market, By Type
- Australia
- Australia Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Australia Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Australia Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Australia Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Australia Healthcare Contract Research Organizations (CRO) Market, By Type
- Thailand
- Thailand Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Thailand Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Thailand Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Thailand Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Thailand Healthcare Contract Research Organizations (CRO) Market, By Type
- Taiwan
- Taiwan Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Taiwan Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Taiwan Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Taiwan Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Taiwan Healthcare Contract Research Organizations (CRO) Market, By Type
- Indonesia
- Indonesia Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Indonesia Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Indonesia Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Indonesia Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Indonesia Healthcare Contract Research Organizations (CRO) Market, By Type
- Malaysia
- Malaysia Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Malaysia Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Malaysia Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Malaysia Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Malaysia Healthcare Contract Research Organizations (CRO) Market, By Type
- Singapore
- Singapore Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Singapore Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Singapore Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Singapore Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Singapore Healthcare Contract Research Organizations (CRO) Market, By Type
- Asia Pacific Healthcare Contract Research Organizations (CRO) Market, By Type
- Latin America
- Latin America Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Latin America Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Latin America Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Latin America Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Brazil
- Brazil Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Brazil Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Brazil Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Brazil Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Brazil Healthcare Contract Research Organizations (CRO) Market, By Type
- Argentina
- Argentina Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Argentina Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Argentina Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Argentina Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Argentina Healthcare Contract Research Organizations (CRO) Market, By Type
- Colombia
- Colombia Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Colombia Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Colombia Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Colombia Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Colombia Healthcare Contract Research Organizations (CRO) Market, By Type
- Chile
- Chile Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Chile Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Chile Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Chile Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Chile Healthcare Contract Research Organizations (CRO) Market, By Type
- Latin America Healthcare Contract Research Organizations (CRO) Market, By Type
- Middle East and Africa (MEA)
- Middle East and Africa Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Middle East and Africa Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Middle East and Africa Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Middle East and Africa Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- South Africa
- South Africa Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- South Africa Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- South Africa Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- South Africa Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- South Africa Healthcare Contract Research Organizations (CRO) Market, By Type
- Saudi Arabia
- Saudi Arabia Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Saudi Arabia Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Saudi Arabia Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Saudi Arabia Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Saudi Arabia Healthcare Contract Research Organizations (CRO) Market, By Type
- UAE
- UAE Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- UAE Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- UAE Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- UAE Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- UAE Healthcare Contract Research Organizations (CRO) Market, By Type
- Kuwait
- Kuwait Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Kuwait Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Kuwait Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Kuwait Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Kuwait Healthcare Contract Research Organizations (CRO) Market, By Type
- Egypt
- Egypt Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Egypt Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Egypt Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Egypt Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Egypt Healthcare Contract Research Organizations (CRO) Market, By Type
- Israel
- Israel Healthcare Contract Research Organizations (CRO) Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-Clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
- Drug Discovery
- Israel Healthcare Contract Research Organizations (CRO) Market, By Service
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/ Assurance
- Bio-statistics
- Investigator Payments
- Laboratory
- Sterility Testing
- Container/Closure Testing
- Extractables and Leachable Testing
- Environmental Monitoring (Including Microbiology Testing)
- Disinfectant Efficacy Studies
- Others
- Patient And Site Recruitment
- Technology
- Others
- Israel Healthcare Contract Research Organizations (CRO) Market, By Therapeutic Area
- Oncology
- CNS Disorders
- Infectious Diseases
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Diseases
- Diabetes
- Ophthalmology
- Pain Management
- Others
- Israel Healthcare Contract Research Organizations (CRO) Market, By Molecule
- Pharmaceutical
- Small Molecules
- Biologics
- Medical Device
- Israel Healthcare Contract Research Organizations (CRO) Market, By Type
- Middle East and Africa Healthcare Contract Research Organizations (CRO) Market, By Type
- North America
Report content
Qualitative Analysis
- Industry overview
- Industry trends
- Market drivers and restraints
- Market size
- Growth prospects
- Porter’s analysis
- PESTEL analysis
- Key market opportunities prioritized
- Competitive landscape
- Company overview
- Financial performance
- Product benchmarking
- Latest strategic developments
Quantitative Analysis
- Market size, estimates, and forecast from 2018 to 2030
- Market estimates and forecast for product segments up to 2030
- Regional market size and forecast for product segments up to 2030
- Market estimates and forecast for application segments up to 2030
- Regional market size and forecast for application segments up to 2030
- Company financial performance
Research Methodology
Grand View Research employs comprehensive and iterative research methodology focused on minimizing deviance in order to provide the most accurate estimates and forecast possible. The company utilizes a combination of bottom-up and top-down approaches for segmenting and estimating quantitative aspects of the market. In Addition, a recurring theme prevalent across all our research reports is data triangulation that looks market from three different perspectives. Critical elements of methodology employed for all our studies include:
Preliminary data mining
Raw market data is obtained and collated on a broad front. Data is continuously filtered to ensure that only validated and authenticated sources are considered. In addition, data is also mined from a host of reports in our repository, as well as a number of reputed paid databases. For comprehensive understanding of the market, it is essential to understand the complete value chain and in order to facilitate this; we collect data from raw material suppliers, distributors as well as buyers.
Technical issues and trends are obtained from surveys, technical symposia and trade journals. Technical data is also gathered from intellectual property perspective, focusing on white space and freedom of movement. Industry dynamics with respect to drivers, restraints, pricing trends are also gathered. As a result, the material developed contains a wide range of original data that is then further cross-validated and authenticated with published sources.
Statistical model
Our market estimates and forecasts are derived through simulation models. A unique model is created customized for each study. Gathered information for market dynamics, technology landscape, application development and pricing trends is fed into the model and analyzed simultaneously. These factors are studied on a comparative basis, and their impact over the forecast period is quantified with the help of correlation, regression and time series analysis. Market forecasting is performed via a combination of economic tools, technological analysis, and industry experience and domain expertise.
Econometric models are generally used for short-term forecasting, while technological market models are used for long-term forecasting. These are based on an amalgamation of technology landscape, regulatory frameworks, economic outlook and business principles. A bottom-up approach to market estimation is preferred, with key regional markets analyzed as separate entities and integration of data to obtain global estimates. This is critical for a deep understanding of the industry as well as ensuring minimal errors. Some of the parameters considered for forecasting include:
• Market drivers and restrains, along with their current and expected impact
• Raw material scenario and supply v/s price trends
• Regulatory scenario and expected developments
• Current capacity and expected capacity additions up to 2030
We assign weights to these parameters and quantify their market impact using weighted average analysis, to derive an expected market growth rate.
Primary validation
This is the final step in estimating and forecasting for our reports. Exhaustive primary interviews are conducted, on face to face as well as over the phone to validate our findings and assumptions used to obtain them. Interviewees are approached from leading companies across the value chain including suppliers, technology providers, domain experts and buyers so as to ensure a holistic and unbiased picture of the market. These interviews are conducted across the globe, with language barriers overcome with the aid of local staff and interpreters. Primary interviews not only help in data validation, but also provide critical insights into the market, current business scenario and future expectations and enhance the quality of our reports. All our estimates and forecast are verified through exhaustive primary research with Key Industry Participants (KIPs) which typically include:
• Market leading companies
• Raw material suppliers
• Product distributors
• Buyers
The key objectives of primary research are as follows:
• To validate our data in terms of accuracy and acceptability
• To gain an insight in to the current market and future expectations
Data Collection Matrix
|
Perspective |
Primary research |
Secondary research |
|
Supply side |
|
|
|
Demand side |
|
|
Industry Analysis Matrix
|
Qualitative analysis |
Quantitative analysis |
|
|
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."




