
Hairy Cell Leukemia Treatment Market Size, Share & Trends Analysis Report By Treatment (Chemotherapy, Targeted Therapy), By End Use (Hospitals, Specialty Clinics), By Region, And Segment Forecasts, 2024 - 2030
- Report ID: GVR-4-68040-427-6
- Number of Report Pages: 70
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2022
- Forecast Period: 2024 - 2030
- Industry: Healthcare
Market Size & Trends
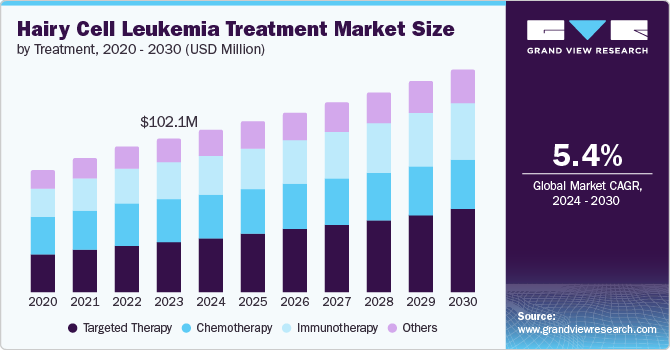
The global hairy cell leukemia treatment market size is estimated at USD 102.1 million in 2023 and is expected to grow at a CAGR of 5.4% from 2024 to 2030. This growth is driven by the increasing incidence of hairy cell leukemia (HCL), targeted therapies advancements, awareness, and improved diagnostics. Hairy cell leukemia is a rare, chronic B-cell lymphoproliferative disorder, accounting for approximately 2% of all leukemia. According to a study published by the National Library of Medicine in September 2023, this disease primarily affects middle-aged to older adults, with a median age of diagnosis of 55 to 60 years, and is more common in men than in females. The median age of patients at diagnosis is 59 years in women and 63 years in men. The prevalence of HCL highlights the importance of specialized treatment options, and recent developments have significantly enhanced patient outcomes.
HCL is a rare type of cancer involving abnormal accumulation of B-lymphocytes within the bone marrow, spleen, and peripheral blood. These atypical cells are referred to as "hairy cells" due to their appearance under a microscope, where they exhibit fine, hair-like projections on their surface. According to the report by the Leukemia & Lymphoma Society, HCL is an incurable form of leukemia, and significant advancements in treatment have greatly extended patient survival. Most individuals with HCL respond well to chemotherapy, particularly with purine analogs like cladribine (Leustatin) and pentostatin (Nipent), which have long been standard treatments. In addition, the FDA has approved moxetumomab pasudotox-tdfk (Lumoxiti) for use in adults with relapsed or refractory HCL, offering an alternative for those who do not respond to initial therapies. These therapeutic advances have played a crucial role in improving outcomes and quality of life for patients with HCL.
The treatment for HCL has undergone a significant transformation with the advent of targeted therapies, which have redefined patient care and expanded the market for HCL treatments. Historically, purine analogs like cladribine and pentostatin have been the foundation of HCL treatment, delivering high response rates and prolonged remissions for many patients. Despite their effectiveness, a subset of patients either do not respond or eventually relapse, underscoring the need for new therapeutic approaches. This unmet need has catalyzed the development of innovative targeted therapies, particularly BRAF inhibitors such as vemurafenib, which have shown remarkable efficacy in patients with the BRAF V600E mutation-a genetic alteration present in nearly all cases of classical HCL.
According to the study published by the National Library of Medicine in May 2021, the clinical success of targeted therapies has been a driving force behind the market growth. For instance, vemurafenib has achieved overall response rates of approximately 91% in relapsed HCL patients, with a complete response rate of 35%, as evidenced by clinical trials and real-world outcomes. These statistics underscore the pivotal role of targeted therapies in improving patient outcomes and expanding the treatment landscape. Moreover, exploration of combination therapies, such as BRAF inhibitors alongside MEK inhibitors or monoclonal antibodies like rituximab, has opened new avenues for treatment. These combinations have shown the potential to deliver more durable responses and overcome resistance mechanisms, which is critical for patients with relapsed or refractory HCL.
Introduction of novel agents like moxetumomab pasudotox, an anti-CD22 recombinant immunotoxin, further demonstrates the innovation in HCL treatment. Designed specifically to target HCL cells, moxetumomab pasudotox represents a new class of therapies that expands the options available for patients, particularly those who have relapsed after traditional treatments. This drug and other emerging therapies have significantly contributed to the diversification and market growth, offering hope to patients with limited options.
Another crucial market driver is the advancement in diagnostic tools and increased awareness among healthcare professionals. Adoption of technologies like flow cytometry, immunophenotyping, and molecular testing for BRAF mutations has revolutionized diagnosis of HCL. These tools enable earlier and more accurate diagnosis, which is critical for effective treatment planning and improving patient prognoses. Early diagnosis allows for timely intervention, leading to better disease management and enhanced quality of life for patients. As more patients are accurately diagnosed and treated, the market for HCL treatments continues to expand, driven by increased identification of HCL cases and the corresponding demand for effective therapies.
Market Concentration & Characteristics
The HCL treatment market is experiencing a surge of innovation fueled by advancements in targeted therapies and personalized medicine. Resistance to existing treatments in some patients has spurred the development of new therapeutic strategies, such as BRAF inhibitors, which have become a key focus. Further, research into combination therapies-like pairing BRAF inhibitors with MEK inhibitors or monoclonal antibodies-promises to deliver more sustained responses and combat resistance. In addition, introducing novel agents like moxetumomab pasudotox, an anti-CD22 recombinant immunotoxin, is paving the way for new treatment classes, specifically targeting unique cell markers associated with HCL. These advancements are revolutionizing the HCL treatment landscape, providing patients with more effective and personalized options, and propelling market growth.
Mergers and acquisitions (M&A) have been pivotal in the market growth and consolidation. The pharmaceutical industry's focus on oncology, especially in specialized areas like HCL, has led to a wave of strategic acquisitions. Companies developing promising targeted therapies or novel agents for HCL have become prime targets for acquisition as larger pharmaceutical firms aim to strengthen their oncology portfolios with innovative treatments. These M&A activities are driven by the lucrative potential of orphan drug status, which grants market exclusivity, and by strategic acquisition of cutting-edge technologies that enhance broader cancer treatment offerings.
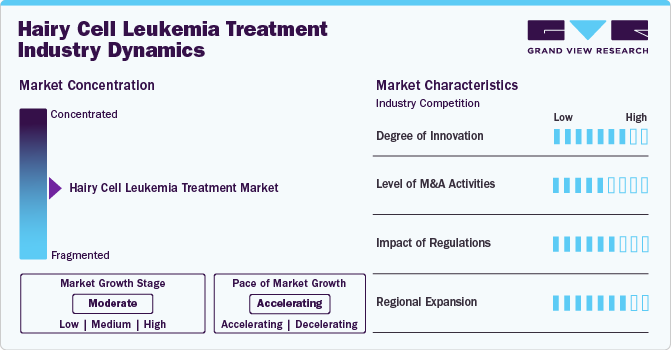
The regulatory framework for Hairy Cell Leukemia (HCL) treatments significantly impacts market development and growth. Due to HCL's rarity, therapies often receive orphan drug designation in the U.S. and Europe, offering incentives like tax credits, extended market exclusivity, and specialized development support. The regulatory landscape has streamlined approval processes for HCL treatments under orphan drug programs, enabling faster market entry for innovative therapies. According to a study published by National Cancer Institute in February 2024, the FDA has not approved oral vemurafenib for relapsed HCL patients, highlighting the complexities of regulatory process. Agencies like FDA and EMA have been instrumental in this process, providing guidance and support in designing and approving clinical trials, particularly for breakthrough therapies. This supportive regulatory framework fosters innovation and ensures that HCL patients have quicker access to new, life-saving treatments, ultimately enhancing the treatment landscape and driving market growth.
The HCL treatment market is experiencing robust regional growth, driven by enhanced healthcare infrastructure, increased awareness, and the rollout of advanced therapies globally. North America leads with high adoption rates and strong FDA support, bolstered by a thriving pharmaceutical sector and R&D ecosystem. Europe mirrors this growth, with the EMA fostering a favorable regulatory environment for new therapies. Expansion into Asia-Pacific and Latin America is accelerating, propelled by improved healthcare access, rising HCL awareness, and significant investment in cancer treatment infrastructure. Countries like China, India, and Brazil are emerging as key markets, with collaborations between global and local pharmaceutical companies further boosting the availability and adoption of HCL treatments.
Treatment Insights
This market is segmented into chemotherapy, targeted therapy, immunotherapy, and others based on treatment. The targeted therapy segment held the largest share of 32.5% in 2023 and is expected to grow at the fastest CAGR of 7.5% during the forecast period. Targeted therapy has revolutionized HCL treatment by offering a more precise and effective approach than traditional chemotherapy. While chemotherapy broadly targets all rapidly dividing cells, often causing significant side effects, targeted therapies specifically attack cancer cells by focusing on distinct molecular targets associated with HCL. This precision results in fewer side effects and significantly improved patient outcomes, making targeted therapy the preferred choice for many. The introduction of purine analogs like cladribine and pentostatin set a new standard of care, providing high response rates and durable remissions. However, the challenge of resistance and relapse in some patients has driven the development of newer therapies like BRAF inhibitors, such as vemurafenib, which are particularly effective in treating patients with the BRAF V600E mutation, found in nearly all classical HCL cases. These advancements underscore the transformative impact of targeted therapies on the HCL treatment landscape, driving market growth and improved patient care.
The emergence of immunotherapy as a potential treatment for HCL is also noteworthy. Although still in the early stages of research, innovative approaches such as immune checkpoint inhibitors and CAR-T cell therapies are showing promising potential. Immune checkpoint inhibitors, which block proteins that prevent the immune system from attacking cancer cells, are evaluated for their ability to enhance the body’s natural defense mechanisms against HCL. Meanwhile, CAR-T cell therapies, which involve engineering a patient’s T cells to recognize better and destroy HCL cells, offer a cutting-edge treatment modality that could significantly improve outcomes for those with resistant or relapsed disease. These advancements in immunotherapy highlight a new era in HCL treatment, providing hope for more effective and targeted therapies that harness the power of immune system to combat this challenging hematologic malignancy.
End Use Insights
Hospitals held the largest share of 64.3% in 2023. Hospitals are typically equipped with advanced diagnostic tools and treatment facilities, essential for managing complex cases of hairy cell leukemia (HCL). They provide a comprehensive range of services, including specialized oncology care, which is crucial for effective treatment of HCL. Furthermore, hospitals offer access to multidisciplinary teams of healthcare professionals, including hematologists, oncologists, and support staff, who are integral to managing disease and addressing any complications. The high volume of patients receiving treatments such as chemotherapy, targeted therapies, and newer immunotherapies in hospital settings further consolidates their significant market share. Moreover, the ability of hospitals to conduct clinical trials and provide cutting-edge treatments contributes to their leading position in the market.
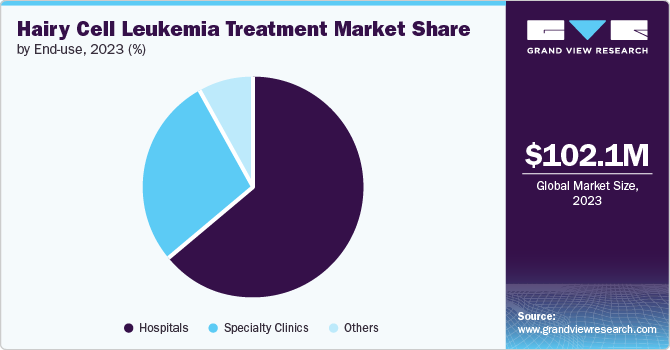
Specialty clinics are projected to experience the fastest CAGR of 6.9% in the market during the forecast period. Specialty clinics offer highly specialized care and advanced treatment options tailored to the unique needs of HCL patients, including cutting-edge therapies and personalized treatment plans. The growing prevalence of HCL and rising demand for expert care are prompting more patients to seek specialized facilities that offer the latest and most effective treatment options. Specialty clinics are also at the forefront of clinical trials and emerging therapies, attracting patients looking for access to innovative treatments that may not be available in general practice settings. Furthermore, specialty clinics' enhanced patient outcomes and ability to provide comprehensive care, including support services and patient education, contribute to their increasing popularity and growth. This shift towards specialized care underscores the evolving landscape of HCL treatment, where expertise and advanced technology are crucial in improving patient outcomes and driving market expansion.
Regional Insights
The hairy cell leukemia treatment market in North America dominated the market in 2023, capturing around 40.0% of total revenue. The region's robust research and development ecosystem drives market growth as pharmaceutical companies invest heavily in developing and refining HCL treatments. Moreover, the prevalence of HCL in North America is relatively higher than in other regions, contributing to substantial market demand.The U.S. and Canada benefit from advanced healthcare infrastructure, high adoption rates of innovative therapies, and strong regulatory support.
U.S. Hairy Cell Leukemia Treatment Market Trends
The hairy cell leukemia treatment market in the U.S. is primarily driven by the high prevalence of the disease and significant investment in medical research. According to the National Cancer Institute, 1,200 to 1,300 new cases are diagnosed each year in the U.S. The market benefits from extensive clinical trial infrastructure and a high level of awareness among healthcare providers, which drives early diagnosis and treatment in this region. U.S. Food and Drug Administration (FDA) provides a supportive regulatory environment that accelerates the approval and availability of cutting-edge therapies.
Europe Hairy Cell Leukemia Treatment Market Trends
The hairy cell leukemia treatment market in Europe is poised for significant growth, driven by increasing healthcare investments and supportive regulatory frameworks. The European Medicines Agency (EMA) facilitates the approval of innovative therapies, such as targeted and immunotherapies, contributing to market growth. Europe has a notable prevalence of HCL, with countries like France, Germany, and the UK showing substantial patient populations. The increasing adoption of advanced therapies and a growing emphasis on personalized treatment plans are key drivers of market expansion.
The UK hairy cell leukemia treatment market is experiencing robust growth driven by increasing demand for advanced therapies and significant improvements in healthcare infrastructure. The National Health Service (NHS) is actively supporting the adoption of innovative treatments, including targeted therapies and immunotherapies, which are becoming more accessible to patients. The UK's esteemed research institutions and participation in international clinical trials are pivotal in advancing new treatment options. According to Blood Cancer UK, approximately 230 new cases of HCL are diagnosed annually, with the disease predominantly affecting older adults. Notably, around 50% of cases are in individuals aged 68 or older, while 25% occur in those under 55. This demographic insight underscores the growing need for specialized treatments and contributes to the market's expansion.
The hairy cell leukemia treatment market in France is expected to grow due to increasing healthcare investments and advancements in treatment options. The French National Agency for the Safety of Medicines and Health Products (ANSM) is crucial in approving new therapies and ensuring their availability to patients. The prevalence of HCL in France supports the demand for specialized treatments, and recent developments include the introduction of advanced targeted therapies and participation in international clinical trials. Moreover, focusing on personalized medicine and innovative treatment approaches drives market growth and improves patient care.
Germany hairy cell leukemia treatment market is experiencing notable growth, fueled by a well-established healthcare system and a robust emphasis on research and development. Germany’s substantial investment in healthcare infrastructure and active participation in European clinical trials significantly contribute to the market's expansion. The Federal Institute for Drugs and Medical Devices (BfArM) plays a crucial role in facilitating the approval and accessibility of new therapies, including targeted treatments and immunotherapies. Despite HCL being rare, with an incidence rate of about 0.3-0.4 cases per 100,000 individuals, it represents approximately 3% of all lymphocytic leukemias. According to Onkopedia, around 70 women and 220 men are diagnosed with HCL annually in Germany. The median age of onset is between 62 and 65 years, with the disease being significantly more common in men across all age groups. This demographic and clinical landscape underscores the ongoing need for advanced treatment options and contributes to market growth.
Asia Pacific Hairy Cell Leukemia Treatment Market Trends
The Hairy Cell Leukemia (HCL) treatment market in the Asia-Pacific region is projected to expand significantly, driven by healthcare infrastructure improvements and increasing HCL awareness. Countries like China, India, and Australia are investing in modernizing their healthcare systems and expanding access to advanced treatments.
Japan hairy cell leukemia treatment market is experiencing notable growth, driven by the nation's advanced healthcare infrastructure and a strong emphasis on research and innovation. Japan's Ministry of Health, Labour, and Welfare (MHLW) plays a crucial role in facilitating the approval and availability of new therapies, including cutting-edge targeted and immunotherapies. The high prevalence of HCL in Japan, particularly the rare Japanese variant (HCL-JV), further propels market expansion. According to the National Library of Medicine, HCL-JV is exceptionally rare, with only 17 documented cases; its presence contributes to the growing demand for specialized treatments. The median age for diagnosis is 75, and men are disproportionately affected, with a sex ratio of 3.2:1. These factors underscore increasing need for advanced, effective treatment options, supporting the overall market growth in Japan.
The hairy cell leukemia treatment market in India is expanding as the country invests in enhancing its healthcare infrastructure and increasing access to advanced treatments. The growing prevalence of HCL and rising awareness among healthcare providers contribute to market growth.
Australia hairy cell leukemia treatment market is experiencing growth due to the country's strong healthcare system and increasing access to advanced therapies. According to Leukaemia Foundation, in 2022, it was projected that 19,403 Australians would be newly diagnosed with blood cancers, including leukemia, lymphoma, and myeloma, highlighting a significant patient base for treatment. The Therapeutic Goods Administration (TGA) plays a pivotal role in this growth by supporting the approval of innovative treatments, such as targeted and immunotherapies.
Latin America Hairy Cell Leukemia Treatment Market Trends
The hairy cell leukemia treatment market in Latin America is experiencing significant growth, primarily driven by increasing awareness of the disease, advancements in treatment options, and a growing patient population. Key governing bodies such as the Brazilian Health Regulatory Agency (ANVISA) and the Pan American Health Organization (PAHO) are instrumental in regulating treatments and ensuring patients access effective therapies.
Brazil hairy cell leukemia treatment market is witnessing robust growth driven by several factors including an increase in the incidence rate of HCL, improved diagnostic techniques, and enhanced access to novel therapies.The Brazilian Ministry of Health plays a crucial role in establishing guidelines for cancer treatment and funding research initiatives aimed at improving patient care. Moreover, public awareness campaigns about blood cancers have led to earlier detection and intervention, further driving market growth.
MEA Hairy Cell Leukemia Treatment Market Trends
The hairy cell leukemia treatment market in the Middle East and Africa (MEA) is witnessing notable growth, fueled by increased awareness of hematological cancers, advancements in healthcare infrastructure, and rising investments in oncology research. While the prevalence of HCL in this region remains lower compared to other cancers, it is gaining more attention due to its distinct characteristics and the specific needs it presents for treatment. According to GLOBOCAN data, leukemia incidence rates in the Middle East and Northern Africa (MENA) region are estimated to be 5.3 per 100,000 among males and 4.0 per 100,000 among females. In addition, a report from the Gulf Cooperation Council highlights leukemia as the fourth most common cancer in this region, underscoring the growing significance of addressing HCL. These factors collectively contribute to the expanding focus on improving treatment options and outcomes for HCL patients in MEA.
Kuwait hairy cell leukemia treatment market is set to grow substantially, driven by a rising incidence rate of hematological malignancies, advancements in medical technology, and increased healthcare expenditure. The Kuwaiti Ministry of Health plays an essential role in regulating cancer treatments while promoting awareness campaigns aimed at early detection among high-risk populations.
Key Hairy Cell Leukemia Treatment Insights
The market is characterized by several key companies dominating the landscape with substantial market share. These companies are leading the industry through technological innovations and rising awareness of treatments.
Key Hairy Cell Leukemia Treatment Companies:
The following are the leading companies in the hairy cell leukemia treatment market. These companies collectively hold the largest market share and dictate industry trends.
- Pfizer Inc
- F. Hoffmann La Roche AG
- Janssen Global Services
- Emcure Pharmaceuticals
- Dr. Reddy’s Laboratories
- Amgen, Inc.
- Hospira
- AbbVie
- AstraZeneca
Hairy Cell Leukemia Treatment Market Report Scope
|
Report Attribute |
Details |
|
Market size value in 2024 |
USD 107.6 million |
|
Revenue forecast in 2030 |
USD 147.6 million |
|
Growth rate |
CAGR of 5.4% from 2024 to 2030 |
|
Historical data |
2018 - 2022 |
|
Forecast period |
2024 - 2030 |
|
Quantitative units |
Revenue in USD million and CAGR from 2024 to 2030 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Treatment, end use, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
|
Country scope |
U.S.; Canada; Mexico; UK; Germany; France; Spain; Italy; Norway; Sweden; Denmark; Japan; China; India; Australia; South Korea; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait |
|
Key companies profiled |
Pfizer Inc; F. Hoffmann La Roche AG; Janssen Global Services; Emcure Pharmaceuticals; Dr. Reddy’s Laboratories; Amgen, Inc.; Hospira; AbbVie; AstraZeneca |
|
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional and segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. Explore purchase options |
Global Hairy Cell Leukemia Treatment Market Report Segmentation
This report forecasts revenue growth at global, regional & country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the hairy cell leukemia treatment market report based on treatment, end use, and region:
-
Treatment Outlook (Revenue, USD Million, 2018 - 2030)
-
Chemotherapy
-
Targeted Therapy
-
Immunotherapy
-
Other
-
-
End Use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Specialty Clinics
-
Other
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Sweden
-
Denmark
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
South Korea
-
Singapore
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
-
Frequently Asked Questions About This Report
b. The global hairy cell leukemia treatment market was valued at USD 102.1 million in 2023 and is expected to reach USD 107.6 million in 2024.
b. The global hairy cell leukemia treatment market is expected to grow at a CAGR of 5.4% from 2024 to 2030 to reach USD 147.6 million by 2030
b. The targeted therapy segment held the largest share of 32.5% in 2023 and is expected to grow at the fastest CAGR of 7.5% during the forecast period.
b. Some prominent players in the market include Pfizer Inc, F. Hoffmann La Roche AG, Janssen Global Services, Emcure Pharmaceuticals, Dr. Reddy’s Laboratories, Amgen, Inc., Hospira, AbbVie, AstraZeneca, and others.
b. This growth is driven by the increasing incidence of hairy cell leukemia, advancements in targeted therapies, and the growing awareness and improved diagnostics. Hairy Cell Leukemia (HCL) is a rare, chronic B-cell lymphoproliferative disorder, accounting for approximately 2% of all leukemia.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."




