
Endometriosis Treatment Market Size, Share & Trends Analysis Report By Treatment Type, By Drug Class, By Route Of Administration, By Distribution Channel, By Region, And Segment Forecasts, 2025 - 2030
- Report ID: GVR-4-68040-005-5
- Number of Report Pages: 150
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2024
- Forecast Period: 2025 - 2030
- Industry: Healthcare
Endometriosis Treatment Market Trends
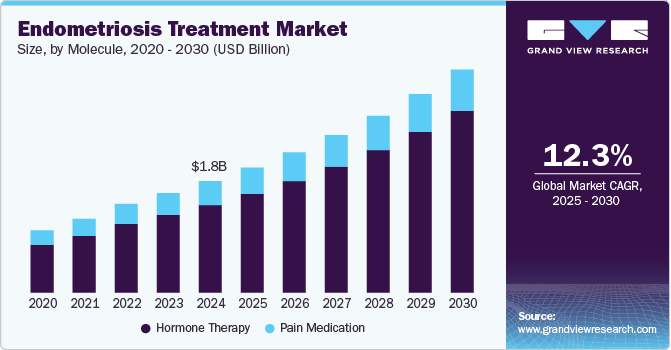
The global endometriosis treatment market size was estimated at USD 1.76 billion in 2024 and is expected to grow at a CAGR of 12.25% from 2025 to 2030. The rising disease burden, increasing disease awareness, and robust product pipeline are among the major factors driving the market growth. According to the World Health Organization (WHO), approximately 10% of reproductive-age women and girls worldwide suffer from endometriosis. In India alone, the disorder affects an estimated 42 million women, as reported by the National Library of Medicine in February 2023. These alarming statistics underscore the urgent need for advanced therapeutic solutions, highlighting the growing market opportunity for endometriosis treatments.
Pharmaceutical companies are actively addressing this rising disease burden by introducing novel therapies aimed at improving patient outcomes. For instance, in August 2022, Pfizer Inc. and Myovant Sciences announced the U.S. FDA approval of MYFEMBREE, an oral treatment specifically designed to alleviate moderate to severe pain associated with endometriosis in premenopausal women. This development represents a significant step forward in addressing the unmet medical needs of patients suffering from this condition.
Awareness campaigns organized by public health departments and non-profit organizations also play a crucial role. Government support for women's health and specific guidelines for endometriosis care further contribute to heightened awareness. For example, in February 2022, the European Society of Human Reproduction and Embryology (ESHRE) published comprehensive guidelines on care. These guidelines provided best practices for managing the condition, including treatment recommendations and diagnostic approaches to alleviate pain and address infertility caused by endometriosis.
The endometriosis treatment industry is poised for significant growth due to increasing funding for research and development. Governments, private organizations, and pharmaceutical companies are making substantial investments to address the rising prevalence of endometriosis and the need for effective treatments. For instance, in March 2023, the Scottish Government and Wellbeing of Women awarded approximately USD 302.5 million to researchers in Scotland and England to explore the potential of dichloroacetate as a treatment for the condition. Additionally, the UK-based charity Wellbeing of Women had invested over USD 1,197.9 million in endometriosis research by March 2023, underscoring the growing focus on developing innovative solutions for this condition.
Market Concentration & Characteristics
The global endometriosis treatment market demonstrates a high degree of innovation, driven by advancements in drug discovery, minimally invasive therapies, and hormonal treatment developments. Key areas of focus include next-generation GnRH (Gonadotropin-releasing Hormone) antagonists, personalized medicine approaches, and non-hormonal therapies targeting the inflammatory pathways associated with endometriosis. Continuous investment in R&D has resulted in improved symptom management and reduced recurrence rates, significantly enhancing patient quality of life and driving market growth.
The global endometriosis treatment market experiences a moderate to robust level of mergers and acquisitions (M&A), as pharmaceutical giants and specialty biotech firms focus on expanding their women’s health portfolios. Recent trends include the acquisition of innovative pipeline candidates targeting endometriosis pain and inflammation, as well as partnerships aimed at co-developing next-generation therapies. Strategic collaborations with medical device companies for adjunctive surgical solutions are also on the rise, enhancing the overall treatment landscape.
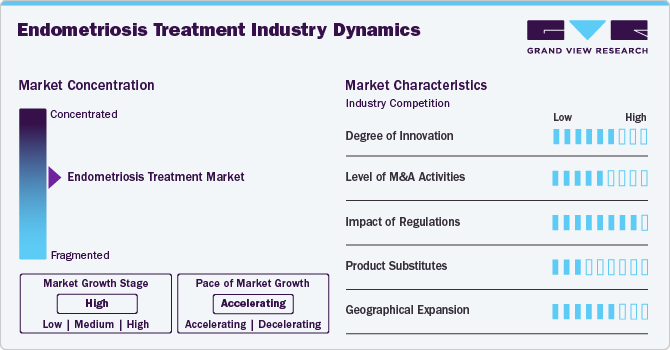
Regulatory frameworks heavily influence the endometriosis treatment market. The FDA plays a pivotal role in approving novel therapies, particularly through expedited pathways like Breakthrough Therapy Designation and Fast Track for high-need indications. However, scrutiny over drug pricing and reimbursement challenges for specialty therapies pose hurdles for manufacturers. Legislative efforts, such as the Inflation Reduction Act, aim to enhance drug affordability, potentially affecting the pricing dynamics in the segment.
The threat of product substitutes in the endometriosis treatment market includes surgical interventions such as laparoscopy and hysterectomy, which are often used in conjunction with or as an alternative to pharmacological treatments. Additionally, lifestyle interventions such as dietary management and physical therapy are gaining traction among patients seeking holistic care. Despite these alternatives, branded pharmaceuticals, particularly GnRH antagonists and hormonal therapies, maintain a strong market position due to their efficacy and established clinical outcomes.
Companies in the global endometriosis treatment market are strategically focusing on expanding their presence through improved patient outreach in both rural and urban areas. Telemedicine and digital health platforms are being leveraged to enhance diagnosis rates and ensure timely initiation of treatment. Collaborations with health systems and payers are critical for improving drug affordability and addressing the significant unmet need for accessible and effective care.
Treatment Type Insights
Hormone therapy dominated the endometriosis treatment market with a revenue share of 78.51% in 2024, driven by increasing awareness and diagnosis rates. Several FDA-approved hormonal treatments have demonstrated efficacy in treating the condition. Gonadotropin-releasing hormone (GnRH) agonists, such as leuprolide acetate, are widely used to create a hypoestrogenic state, reducing lesion activity.
The pain Medication segment is expected to grow at a substantial growth rate over the projected period. Pain medication plays a pivotal role in managing the condition, a chronic condition characterized by the growth of endometrial-like tissue outside the uterus, often causing debilitating pain and discomfort. For many patients, pain relief is the primary focus of treatment, especially when surgical or hormonal therapies are not suitable or as an adjunct to these methods. Effective pain management not only improves the quality of life for those affected but also allows them to maintain daily activities and overall well-being.
Drug Class Insights
Gonadotropin releasing hormone dominated the overall market with a revenue share of 51.33% in 2024 and is expected to grow at a fastest CAGR of the forecast period. GnRH therapies in endometriosis treatment presents substantial opportunities. Increasing awareness, a growing prevalence of the condition, and the demand for minimally invasive solutions drive innovation. Advances in drug formulations, such as oral GnRH antagonists, and expanding indications in different patient demographics offer lucrative potential. As researchers explore combination therapies and novel delivery systems, GnRH-based treatments continue to hold a prominent position in this evolving therapeutic landscape.
Oral contraceptives play a crucial role in the management of endometriosis, offering a first-line treatment option for many patients. These medications work by regulating hormone levels, particularly estrogen and progesterone, to suppress ovulation and reduce the growth of endometrial tissue. This helps alleviate common symptoms like pelvic pain and irregular menstruation, improving the quality of life for individuals affected by the condition.
Route of Administration Insights
The oral segment dominated the overall market in 2024. Oral medications play a pivotal role in the management of endometriosis, offering convenience, accessibility, and effective symptom control for patients. These treatments are particularly valuable for individuals seeking non-invasive options to manage chronic pain, inflammation, and menstrual irregularities caused by endometriosis. By targeting hormonal imbalances, oral drugs can significantly reduce the recurrence of lesions and enhance the quality of life for patients.
Injectable is expected to grow at the fastest CAGR over the forecast period. Primary uses of injections in endometriosis treatment involve the administration of GnRH agonists and antagonists. These medications are powerful tools for regulating hormonal fluctuations and managing endometriosis-related pain and inflammation. By delivering these drugs via injection, healthcare providers can ensure precise and controlled dosing, which is crucial in achieving the desired therapeutic effects. Furthermore, injectable progestins and other hormonal medications are used in treatment. These injections offer a sustained and consistent release of hormones, which can be advantageous in managing symptoms.
Distribution Channel Insights
The retail pharmacies segment dominated the overall market in 2024 and is expected to grow at a CAGR of 11.84% over the forecast period. Retail pharmacies play a crucial role in the management and treatment of endometriosis by providing accessible points of care for patients. With the advent of new medications and treatments, retail pharmacies offer convenience, affordability, and widespread availability of essential drugs for managing chronic conditions. Notable pharmacy chains such as CVS Health, Walgreens Boots Alliance, Rite Aid, and Walmart Pharmacy ensure that patients can easily access prescribed medications, including hormonal therapies and pain relievers, which are key in treating endometriosis.
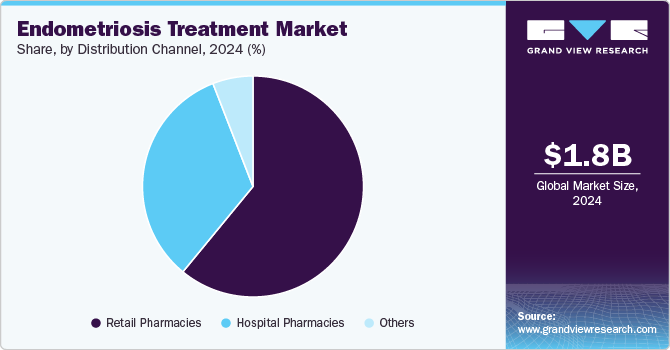
Others segment, which include mail-order pharmacies, specialty clinics, and online drug stores, is expected to grow at the fastest CAGR over the forecast period. This segment is particularly significant as it caters to patients requiring personalized care plans, rare medication formulations, and specialized delivery mechanisms. The availability of compounded medications tailored to individual hormonal needs underscores the importance of other pharmacies in enhancing therapeutic outcomes.
Regional Insights
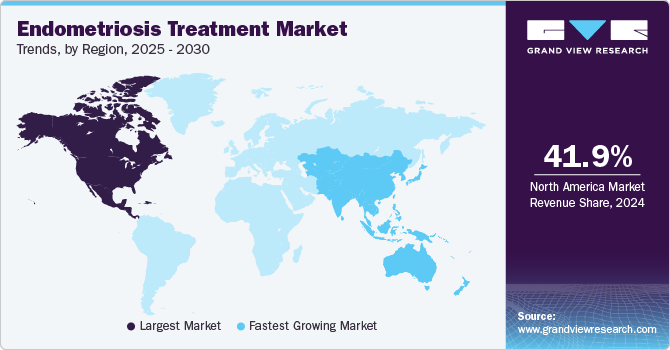
North America endometriosis treatment market dominated the global market, holding a share of 41.87% in 2024. This dominance is attributed to a robust healthcare infrastructure, high awareness about women's health, and a strong presence of pharmaceutical companies actively developing and marketing endometriosis drugs. The availability of advanced hormonal therapies, surgical interventions, and non-hormonal treatment options has contributed to the market's growth. Additionally, government initiatives aimed at improving women’s health and early diagnosis are driving the adoption of effective treatment solutions across hospitals, clinics, and specialty centers.
U.S. Endometriosis Treatment Market Trends
The U.S. endometriosis treatment market captured a significant share of the North America region in 2024, driven by technological innovation, regulatory approvals, and research initiatives. These developments aim to address the increasing prevalence of endometriosis and the growing demand for effective treatment options. The market is supported by key players competing to introduce advanced therapies, contributing to the overall market expansion. In August 2023, researchers at the University at Buffalo identified neurotrophins and their receptors as potential targets for addressing pelvic pain. This discovery emphasizes the importance of developing therapies tailored to the root causes of pain, supporting the growing demand for targeted treatments.
Europe Endometriosis Treatment Market Trends
The Europe endometriosis treatment market is expanding steadily across several countries, driven by increased awareness of the disease and improvements in healthcare infrastructure. Countries such as the UK, Germany, France, and Italy are seeing significant growth in the demand for endometriosis treatments. The market’s growth is underpinned by increasing prevalence, improved diagnostic capabilities, and the introduction of new therapeutic options.
The endometriosis treatment market in Germany demonstrates stable growth, supported by advanced healthcare infrastructure and a high prevalence of endometriosis, which drives the demand for effective therapeutic solutions
The UK endometriosis treatment market is exhibiting a consistent growth pattern, supported by advanced healthcare infrastructure, robust pharmaceutical research, and strong collaborations between public and private sectors. Increasing awareness about endometriosis and its impact on women’s health has led to improved diagnostic rates and a growing demand for effective therapeutic options
The endometriosis treatment market in France is growing, driven by government initiatives, a supportive regulatory framework, and the presence of leading research institutions. The market benefits from comprehensive national strategies that prioritize awareness, early diagnosis, treatment advancements, and research support.
Asia-Pacific Endometriosis Treatment Market Trends
The Asia-Pacific endometriosis treatment market is expanding rapidly at a CAGR of 13.32% over the forecast period. The demand for endometriosis treatment is increasing due to the rising prevalence of the condition, enhanced awareness, and improvements in healthcare systems. The market is growing as more women seek proper diagnosis and treatment, leading to the development of specialized healthcare centers and treatment options.
The endometriosis treatment market in Japan is experiencing growth, driven by a combination of clinical advancements, supportive government initiatives, and increasing research activities. The country's strong healthcare infrastructure and its focus on women's health contribute significantly to this expansion.
China endometriosis treatment market is expanding due to the country’s large population, a high burden of the disease, favorable government policies, and increasing pharmaceutical innovations. The market is significantly driven by the efforts of both local and international pharmaceutical companies focused on developing novel therapies to address endometriosis-associated pain.
Latin America Endometriosis Treatment Market Trends
The endometriosis treatment market in Latin America is experiencing growth, driven by increasing awareness of the disease, improved healthcare access, and ongoing medical advancements. Key countries such as Brazil, Mexico, and Argentina are leading the expansion.
The Brazil endometriosis treatment market is expected to grow due to increasing awareness of women’s health issues, rising prevalence of endometriosis, and government support. Brazil has a large population of women of reproductive age, contributing to the high burden of endometriosis and related health conditions.
Middle East & Africa Endometriosis Treatment Market Trends
The endometriosis treatment market in the Middle East and Africa (MEA) is in its nascent stages, with limited awareness and access to healthcare in many regions. However, countries such as Saudi Arabia, the UAE, and South Africa are seeing gradual growth due to rising awareness, government healthcare initiatives, and improving healthcare infrastructure.
South Africa endometriosis treatment market is projected to experience growth due to rising awareness, increasing healthcare investments, and the growing recognition of endometriosis as a critical health issue.
Key Endometriosis Treatment Company Insights
Key market players are focusing on launching innovative products, and adopting various growth strategies, and technological advancements. Several market players are acquiring smaller players to strengthen their market positions. This strategy enables companies to increase their capabilities, expand product portfolios, and improve competencies. Some key players adopting this strategy are Teva Pharmaceutical Industries Ltd. and AbbVie, Inc.
Key Endometriosis Treatment Companies:
The following are the leading companies in the endometriosis treatment market. These companies collectively hold the largest market share and dictate industry trends.
- Bayer AG
- Pfizer, Inc.
- AbbVie, Inc
- AstraZeneca
- ObsEva SA
- Teva Pharmaceutical Industries Ltd.
- Zydus Healthcare Limited
- Astellas Pharma, Inc.
- Gedeon Richter Plc.
- Takeda Pharmaceutical Company Limited
View a comprehensive list of companies in the Endometriosis Treatment Market
Recent Developments
-
In June 2024, PHOENIX Group entered into a Reduced Wholesale Model agreement with AstraZeneca for the distribution of its portfolio of medicines, including Zoladex, in the United Kingdom. This strategic partnership aims to enhance the availability and distribution of AstraZeneca's treatments to healthcare providers across the region.
-
In February 2024, ObsEva SA and Theramex entered into a strategic licensing agreement to support the commercialization and expansion of Linzagolix across the global market. Linzagolix is being developed as a potential treatment for endometriosis-associated pain.
-
In January 2024, Hera Biotech, Inc., a Texas-based biotechnology company specializing in tissue-based diagnostics for endometriosis, announced its acquisition of Scailyte AG, a Swiss firm known for its expertise in single-cell omics and AI-driven biomarker discovery. This strategic move aims to accelerate the development of non-invasive clinical diagnostic tests for endometriosis, enhancing early detection and personalized treatment options in the U.S. market.
Endometriosis Treatment Market Report Scope
|
Report Attribute |
Details |
|
Market size value in 2025 |
USD 1.97 billion |
|
Revenue forecast in 2030 |
USD 3.52 billion |
|
Growth rate |
CAGR of 12.25% from 2025 to 2030 |
|
Actual data |
2018 - 2024 |
|
Forecast period |
2025 - 2030 |
|
Quantitative units |
Revenue in USD million/billion and CAGR from 2025 to 2030 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Treatment type, drug class, route of administration, distribution channel, region |
|
Country scope |
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; South Korea; Australia; Brazil; Argentina; South Africa; UAE; Saudi Arabia; Kuwait |
|
Key companies profiled |
Bayer AG; Pfizer, Inc.; AbbVie, Inc.; AstraZeneca; ObsEva SA; Teva Pharmaceutical Industries Ltd.; Zydus Healthcare Limited; Astellas Pharma, Inc.; Gedeon Richter Plc.; Takeda Pharmaceutical Company Limited. |
|
Customization scope |
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. Explore purchase options |
Global Endometriosis Treatment Market Report Segmentation
This report forecasts revenue growth at global, regional, and contry levels, and provides an analysis of the latest trends in each of the sub-segments from 2018 to 2030. For the purpose of this report, Grand View Research has segmented the global endometriosis treatment market report based on the treatment type, drug class, route of administration, distribution channel, and region:
-
Treatment Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Pain Medication
-
Hormone Therapy
-
-
Drug Class Outlook (Revenue, USD Million, 2018 - 2030)
-
NSAIDs
-
Oral Contraceptive
-
Gonadotropin Releasing Hormone
-
Others
-
-
Route Of Administration Outlook (Revenue, USD Million, 2018 - 2030)
-
Oral
-
Injectable
-
Others
-
-
Distribution Channel Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospital Pharmacy
-
Retail Pharmacy
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
Germany
-
U.K.
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
India
-
Japan
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global endometriosis treatment market is expected to grow at a compound annual growth rate of 12.25% from 2025 to 2030 to reach USD 3.52 billion by 2030.
b. North America dominated the endometriosis treatment market and accounted for the largest revenue share of 41.87% in 2024. This dominance is attributed to a robust healthcare infrastructure, high awareness about women's health, and a strong presence of pharmaceutical companies actively developing and marketing endometriosis drugs.
b. Some key players operating in the endometriosis treatment market include Bayer AG; Pfizer, Inc.; AbbVie, Inc.; AstraZeneca; ObsEva SA; Teva Pharmaceutical Industries; Zydus Healthcare Limited; Eli Lilly and Company; and Astellas Pharma Inc.
b. Key factors that are driving the endometriosis treatment market growth include a robust late stage product pipeline, rising prevalence of endometriosis, and increasing awareness about women’s health
b. The global endometriosis treatment market size was estimated at USD 1.76 billion in 2024 and is expected to reach USD 1.97 billion in 2025.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."




