
STD Diagnostics Market Size, Share & Trends Analysis Report By Application (HIV Testing, HSV Testing, Chlamydia Testing), By Product, By Technology, By Location Of Testing, By Region, And Segment Forecasts, 2025 - 2030
- Report ID: 978-1-68038-686-8
- Number of Report Pages: 156
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
STD Diagnostics Market Size & Trends
The global STD diagnostics market size was estimated at USD 10.63 billion in 2024 and is expected to grow at a CAGR of 7.1% from 2025 to 2030. The market is primarily influenced by advancements in diagnostic technology and increased government efforts to promote early detection and treatment. The growing burden of STDs serves as a key driver for the industry. This growth can also be attributed to rapid technological advancements, and high R&D investments by key players to introduce novel & innovative products such as genetic biomarkers for antibiotic-resistant strains of bacteria, multiplex Point-of-Care (PoC), & self-testing products in the market.

Technological advancements related to accuracy, portability, and cost-effectiveness are expected to drive the Sexually Transmitted Disease (STD) diagnostics market over the forecast period. Key players are expanding their test portfolios by investing in R&D to develop diagnostic kits that can identify emerging diseases or by entering into agreements with other kit manufacturing companies. For instance, in June 2023, Linear Diagnostics secured funding to create an STI rapid test. The UK-based company, a spinout from the University of Birmingham, received support from the UK's National Institute for Health and Care Research (NIHR). The funding aims to facilitate the development of a 20-minute test for diagnosing gonorrhoea and Chlamydia, addressing the need for faster and more accessible STI diagnostics.

Furthermore, in the ever-evolving landscape of STD diagnostics, key trends point towards integrating advanced technologies, contributing significantly to market growth. The introduction of sophisticated tools, such as multiplex PCR products and next-generation HIV testing kits, stands out as a pivotal development. Companies such as Seegene, with products like Anyplex, Allplex, and Seeplex, have played a crucial role in expanding diagnostic capabilities. For instance, the Allplex STI/BV Panel Assay can distinguish among 28 different causative agents in a single test, showcasing the substantial progress made in diagnostic precision. This technological advancement not only broadens the spectrum of detectable sexually transmitted infections but also facilitates more accurate and targeted treatments.
Better reimbursement facilities overcome the high pricing issue to some extent in developed regions such as the U.S. and Europe. According to WHO, about 50% of healthcare financing in low-income countries comes from out-of-pocket payments, compared to ~14% in high-income countries and ~30% in middle-income countries. When payments from public health insurance, general government expenditure, and prepaid private insurance are combined, approximately 38% of healthcare financing in low-income countries is combined in funding pools, which allow the risks of healthcare costs to be shared across population groups, as compared to 80% in high-income countries and approximately 60% in middle-income countries.
Market Dynamics
The emphasis on diversified diagnostic capabilities clearly reflects the industry's commitment to enhancing healthcare outcomes and addressing the complexities associated with the diagnosis of STIs. As these advancements become more widely adopted, the STD diagnostics market is poised for further growth, driven by a commitment to effective and comprehensive diagnostic solutions.
Governments of developed economies are undertaking initiatives to promote the early diagnosis of STDs. The Sexually Transmitted Infections National Strategic Plan is a groundbreaking, first-of-its-kind five-year plan aimed at reversing the recent rapid growth in STIs in the U.S. The STI Plan establishes a vision, objectives, goals, and methods for dealing with the STI crisis. Indicators with measurable targets are also included to track progress. The STI Plan focuses on gonorrhea, chlamydia, syphilis, and HPV, which have the highest morbidity rates, the most persistent & pervasive STI inequities, and the biggest impact on the health of the people, according to the U.S. national data. This national plan focuses on increasing awareness about STIs and expanding high-quality, affordable screening, diagnosis, and treatment.

However, the number of government initiatives is increasing to contain the STD epidemic in various regions. These initiatives include regular vaccination coverage for hepatitis A, hepatitis B, human papillomavirus, and herpes zoster virus. According to the CDC, January 2023 data, the HPV vaccine can aid in the prevention of 32,100 cases of cancer every year. In the U.S., vaccination coverage for herpes zoster among adults aged 50 & above and adults aged 60 & above was 24.1% and 34.5%, respectively. Similarly, the vaccination coverage of HBV was around 67% in 2018, below the Healthy People 2020 target of 90%, but it still acts as a restraining factor for market growth. HPV vaccination increased from 2.1% in 2011 to 26.3% in 2018 among men and from 20.7% in 2010 to 52.8% in 2018 among women aged 19-26.
Market Concentration & Characteristics
The market shows a high degree of innovation, driven by molecular technologies, AI-based tools, and home-testing kits. Companies are developing faster, more accurate, and user-friendly solutions. Innovations like CRISPR diagnostics and digital health platforms are redefining detection methods, emphasizing patient privacy, real-time monitoring, and accessibility, especially critical in underserved and emerging markets.
Mergers and acquisitions are increasing as larger players seek to expand their diagnostic portfolios. Companies acquire smaller innovators specializing in molecular diagnostics, AI integration, or point-of-care technologies. This consolidation helps accelerate innovation adoption, expand geographical reach, and compete effectively against emerging startups in a rapidly evolving market with growing demand for faster and decentralized testing.

Regulatory frameworks significantly influence product development and market entry. Agencies demand higher sensitivity, faster turnaround, and strong data protection. Self-testing devices face rigorous scrutiny to ensure reliability. Global harmonization of standards is slowly improving, but varying regional requirements continue challenging companies, particularly those aiming for multinational expansion and rapid launch of innovative diagnostics.
Companies are broadening their STD diagnostics portfolios by adding multiplex testing kits, home-testing solutions, and AI-enabled digital health platforms. Expansion focuses on tests detecting multiple infections simultaneously, such as HIV, HPV, chlamydia, and gonorrhea, to meet growing demand for comprehensive, user-friendly, and cost-effective solutions that can be used both in clinics and at home.
Emerging markets present significant opportunities, particularly in Asia Pacific, Latin America, and Africa. Companies invest heavily in regional partnerships, localized manufacturing, and distribution networks. Efforts focus on affordability, awareness campaigns, and overcoming stigma barriers. North America and Europe continue to lead in technology adoption, but high growth rates are seen in underserved global regions.
Product Insights
The consumables segment dominated the market in 2024 with a revenue share of 70.01% and is anticipated to grow at the fastest CAGR over the forecast period, owing to increased demand for consumables coupled with the introduction of innovative solutions. Increasing product approvals for addressing the high demand for diagnosis of STDs may contribute to the growth of respective consumables in the market. For instance, in May 2022, Abbott’s Alinity m STI Assay received FDA clearance. It is a multiplex test that can detect and differentiate four common STDs simultaneously-Neisseria gonorrhoeae (NG), Chlamydia trachomatis (CT), Mycoplasma genitalium (MG), and Trichomonas vaginalis (TV). In addition, companies such as Ortho-Clinical Diagnostics, Inc. recently received approval for the VITROS Immunodiagnostic HIV Combo Reagent Pack.
Furthermore, the instruments and services segment within the market is experiencing robust growth, propelled by technological advancements and the increasing global burden of sexually transmitted infections. Laboratory instruments such as PCR thermal cyclers, flow cytometers, and lateral flow readers are essential for precise and efficient STD detection, particularly in centralized laboratory settings. Simultaneously, point-of-care (POC) devices, including portable rapid diagnostic kits and microfluidic-integrated phone chips, are gaining traction due to their ability to deliver quick results in decentralized locations, enhancing accessibility and timely treatment. The services component, encompassing laboratory testing and diagnostic services, is expanding in response to the growing demand for early and accurate STD detection. This expansion is further supported by increased investments in healthcare infrastructure and public health initiatives aimed at controlling the spread of STDs. Collectively, these factors contribute to the significant growth of the Instruments and Services market segment.
Application Insights
The HIV segment dominated the market with a share of 32.24% in 2024 and is anticipated to witness the fastest growth at a CAGR over the forecast period, owing to high testing rates, increased product approvals, such as fourth-generation HIV tests & self-testing kits, and significant R&D initiatives relevant to novel products. In March 2023, the CDC announced the launch of a Together TakeMeHome (TTMH) project to distribute up to 1 million HIV self-tests over 5 years. These tests can be ordered by people aged 17 years and older in the U.S., including Puerto Rico, via an online portal. The CDC develops the test in partnership with Building Healthy Online Communities (BHOC), OraSure Technologies, Emory University, Signal Group, and NASTAD.
The HPV testing segment is expected to grow at a significant CAGR during the forecast period. Traditionally, the Pap smear has been the primary screening method for cervical cancer. However, there has been a shift towards HPV testing as a more sensitive and reliable method for detecting high-risk HPV strains that are associated with the development of cervical cancer. New product launches specifically by a reputed organization such as UNICEF play an important role in creating awareness of cervical cancer. In January 2023, UNICEF launched a new comprehensive cervical cancer toolkit, including an HPV DNA-based diagnostic tool, a preventive HPV vaccine, and a treatment pool. Launching this kit will benefit women in lower-income countries.
Technology Insights
The immunoassay segment dominated the market in 2024 with a revenue share of 44.37%, owing to higher testing rates for Sexually Transmitted Disease (STD) diagnostics. Moreover, the approval of new immunoassay products for the diagnosis of sexually transmitted diseases may impel market growth. For instance, in August 2020, DiaSorin, Inc. received 510 (k) approval from the U.S. FDA for introducing LIAISON XL MUREX anti-HBe, which is used to diagnose HBV infection.
The molecular diagnostics segment is expected to exhibit the fastest growth rate over the forecast period. An increase in the use of high-throughput PCR technology to detect Sexually Transmitted Diseases (STDs) is expected to drive the NAAT segment. Advantages such as cost-effectiveness, user-friendliness, and accuracy are estimated to increase the adoption of this technology. Most of the urine tests for STDs are performed using NAATs owing to their high sensitivity. In January 2025, Roche announced that the U.S. Food and Drug Administration (FDA) had granted 510(k) clearance and a Clinical Laboratory Improvement Amendments of 1988 (CLIA) waiver for its cobas liat sexually transmitted infection (STI) multiplex assay panels. These panels-covering chlamydia and gonorrhea (CT/NG), as well as chlamydia, gonorrhea, and Mycoplasma genitalium (CT/NG/MG)-enable clinicians to diagnose and distinguish between multiple STIs using a single patient sample.
Location of Testing Insights
The laboratory testing segment dominated the market with a share of 78.36% in 2024, driven by a high market penetration rate and procedure volumes. The presence of extensive ancillary support with respect to manpower and infrastructure is also a key driver. Many healthcare institutions are working with laboratories to integrate different tests. Public health laboratories are important in performing high-quality testing for STDs and associated antimicrobial resistance. According to the Public Health England report, in 2023, the number of sexual health screenings-including diagnostic tests for chlamydia, gonorrhoea, syphilis, and HIV-increased by 8.3%, rising from 2,177,325 in 2022 to 2,358,987. New STI diagnoses grew 4.7% during the same period, from 383,789 to 401,800. The most frequently diagnosed STIs in 2023 were chlamydia (194,970 cases, accounting for 48.5% of all new STI cases), gonorrhoea (85,223 cases, 21.2%), first-episode genital herpes (27,167 cases, 6.8%), and first-episode genital warts (26,133 cases, 6.5%).

The point-of-care testing segment is estimated to witness the fastest growth over the forecast period. There is an increasing interest in developing such products owing to shorter turnaround times, enabling healthcare providers to make treatment decisions at clinicians’ offices promptly. Therefore, various companies are designing assays & molecular diagnostic platforms for POC or near-patient testing. For instance, digene HC2 CT-GC Dual ID DNA Test, digene HC2 GC-ID DNA Test for the diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis by QIAGEN and ResistancePlus MG FleXible for diagnosing Mycoplasma genitalium with macrolide-resistance detection by Danaher Corporation (Cepheid) are among such products.
Regional Insights
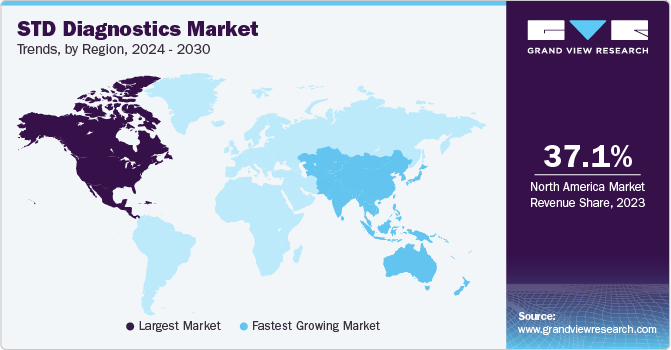
North America held the largest market share in 2024 and is expected to maintain its dominance over the forecast period. This dominance can be attributed to the high testing rates, technological advancements, proactive government measures, improvements in healthcare infrastructure, and the presence of major players. Reimbursement policies and favorable government initiatives are likely to drive market growth. The CMS has introduced the New Technology Add-on Payment (NTAP) program to reduce hospital financial losses. An increase in the number of partnerships between in-vitro diagnostic companies and non-profit organizations for spreading awareness about STDs is creating lucrative growth opportunities in the STD diagnostics industry.
U.S. STD Diagnostics Market Trends
The STD diagnostics market in the U.S. is experiencing significant growth, driven by rising infection rates, increased public awareness, and advancements in diagnostic technologies. This growth is fueled by factors such as the increasing prevalence of STDs, government initiatives promoting early detection and treatment, and the adoption of point-of-care testing and self-testing kits. Integrating digital health platforms and telemedicine services also enhances patient access to diagnostic services, expanding the market.
Europe STD Diagnostics Market Trends
The STD diagnostics market in Europe is experiencing steady growth, driven by rising infection rates and increased public health initiatives. This expansion is fueled by the increasing prevalence of sexually transmitted infections (STIs), with over 230,000 chlamydia cases and nearly 97,000 gonorrhoea cases reported in 2023. The demand for advanced diagnostic tools, including point-of-care testing and self-testing kits, is rising to address these public health challenges.
The UK STD diagnostics market is experiencing significant growth driven by several factors: increasing prevalence of STDs, government initiatives promoting regular screening, and advances in diagnostic technologies. Moreover, public awareness campaigns, adopting point-of-care tests, and expanding self-testing kits contribute significantly. The growing number of young adults engaging in at-risk sexual behaviors also drives market demand.
The STD diagnostics market in Germanybenefits from robust healthcare infrastructure, mandatory health insurance, and strong public health policies. In addition, the rising prevalence of STDs and the emphasis on early detection in clinical settings fuel market growth. Technological advancements in diagnostic tools and the increasing use of molecular diagnostics and point-of-care testing drive adoption. Moreover, government support for sexually transmitted infection prevention and control plays a key role.
Asia Pacific STD Diagnostics Market Trends
The STD diagnostics market in Asia Pacific is estimated to witness the fastest growth rate over the forecast period. The high growth rate can be attributed to the rising disease burden of STIs and increasing testing rates. The report released by The Institute for New Era Strategy in August 2023 noted that in 2020, approximately 641,000 individuals in Japan were diagnosed with HCV. Among them, over 530,000 were under the age of 80, and 157,000 were under the age of 60. These findings emphasize the significance of addressing the situation in the STD diagnostic market to ensure effective healthcare responses.
China STD diagnostics market is experiencing significant growth, driven by several key factors. The increasing prevalence of sexually transmitted diseases (STDs), particularly HIV/AIDS, syphilis, and gonorrhea, has heightened the demand for effective diagnostic solutions. Government initiatives to enhance public health awareness and expand access to healthcare services are further propelling market expansion. Technological advancements in diagnostic tools, such as the development of point-of-care (POC) devices and molecular diagnostics, are improving the accuracy and efficiency of STD testing. Furthermore, the rising adoption of home care diagnostics and the expansion of diagnostic centers contribute to market growth. These factors collectively underscore the dynamic market evolution.
The STD diagnostics market in India is experiencing robust growth, driven by several key factors. An expanding middle class with increased disposable income increases demand for healthcare services, including diagnostic testing. Government initiatives such as Ayushman Bharat and the National Health Mission are enhancing healthcare accessibility and affordability, thereby encouraging more individuals to seek diagnostic services. Technological advancements, including integrating artificial intelligence and digital platforms, are improving diagnostic accuracy and efficiency, making testing more reliable and accessible. Moreover, the rise in health awareness and preventive healthcare trends is prompting individuals to opt for regular screenings, further boosting the demand for STD diagnostics. These factors collectively contribute to the significant expansion of the STD diagnostics market in India.
Latin America STD Diagnostics Market Trends
The STD diagnostics market in Latin America is expanding due to several key factors. Technological advancements, such as developing cost-effective and rapid diagnostic tools, have improved accessibility and efficiency in detecting sexually transmitted infections (STIs). Government initiatives, including public-private partnerships, have enhanced healthcare infrastructure and increased STI prevention and treatment awareness. The growing demand for point-of-care testing solutions, which offer quick results and convenience, has also contributed to market growth. The rising prevalence of STIs and the need for early detection further drive the demand for diagnostic services in the region.
Middle East and Africa STD Diagnostics Market Trends
The STD diagnostics market in the Middle East and Africa (MEA) is experiencing significant growth, driven by several key factors. The rising prevalence of sexually transmitted diseases (STDs) in the region has heightened the demand for effective diagnostic solutions. Governments and non-governmental organizations are increasing awareness and implementing public health campaigns to promote early detection and treatment of STDs. Advancements in diagnostic technologies, such as point-of-care testing and self-testing kits, have improved accessibility and convenience for patients.
Key STD Diagnostics Company Insights
Key players are focusing on gaining market approvals for innovative products to diagnose various STDs, regional & product expansions, and collaborations to maintain their share in the market. For instance, in January 2023, Molbio Diagnostics launched the Truenat HSV 1/2 test for herpes simplex virus. With the ability to provide results within an hour, this product significantly boosts the company's STD diagnostic portfolio.
Key STD Diagnostics Companies:
The following are the leading companies in the STD diagnostics market. These companies collectively hold the largest market share and dictate industry trends.
- BD
- F. Hoffmann-La Roche Ltd
- Hologic Inc.
- Abbott
- Cepheid (Danaher)
- Qiagen
- OraSure Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- bioMérieux SA
- Thermo Fisher Scientific, Inc.
- Seegene Inc.
- DiaSorin S.p.A
Recent Developments
-
In March 2025, bioLytical Laboratories Inc. announced the introduction of its INSTI HIV-1/2 Syphilis Test in Australia. This dual HIV-syphilis test delivers results in only 60 seconds, enabling healthcare professionals to diagnose HIV and syphilis quickly and connect patients to care without delay.
-
In January 2025, OraSure Technologies, Inc., announced that the Center for Biologics Evaluation and Research (CBER) of the U.S. Food and Drug Administration (FDA) approved a labeling update for the OraQuick HIV Self-Test. This update broadens the age range of individuals eligible for the test, including adolescents aged 14 and older, whereas it was previously approved for those 17 and older. This change aims to improve access to HIV testing for younger individuals.
-
In February 2023, Mylab introduced a series of rapid tests designed for STD diagnostics. These tests are simple to use, can be stored at room temperature, and are suitable for use at point-of-care facilities in places with limited resources. Moreover, they find utility in blood banks for efficiently identifying transfusion-transmissible infections (TTIs) among blood donors, reducing transmission.
STD Diagnostics Market Report Scope
|
Report Attribute |
Details |
|
Market size value in 2025 |
USD 11.39 billion |
|
Revenue forecast in 2030 |
USD 16.08 billion |
|
Growth rate |
CAGR of 7.1% from 2025 to 2030 |
|
Base year for estimation |
2024 |
|
Historical data |
2018 - 2023 |
|
Forecast period |
2025 - 2030 |
|
Quantitative units |
Revenue in USD million/billion and CAGR from 2025 to 2030 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, trends |
|
Segments covered |
Product, technology, application, location of testing, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
|
Country scope |
U.S.; Canada; Germany; UK; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait |
|
Key companies profiled |
BD; F. Hoffmann-La Roche Ltd.; Hologic, Inc.; bioMérieux SA; Abbott; Cepheid (Danaher Corporation); Qiagen; Seegene Inc.; Thermo Fisher Scientific Inc.; DiaSorin S.p.A.; OraSure Technologies Inc.; Bio-Rad Laboratories, Inc. |
|
Customization scope |
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. Explore purchase options |
Global STD Diagnostics Market Report Segmentation
This report forecasts revenue growth at global, regional, & country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this report, Grand View Research has segmented the global STD diagnostics market based on product, technology, application, location of testing, and region:
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Instruments and Services
-
Consumables
-
Software
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
CT/NG testing
-
Syphilis testing
-
PCR testing
-
Non-PCR testing
-
-
Gonorrhea testing
-
HSV testing
-
PCR testing
-
Non-PCR testing
-
-
HPV testing
-
HIV testing
-
Ureaplasma & Mycoplasma testing
-
Trichomonas
-
VZV testing
-
PCR testing
-
Non-PCR testing
-
-
Others
-
-
Technology Outlook (Revenue, USD Million, 2018 - 2030)
-
Immunoassay
-
Molecular Diagnostics
-
Other
-
-
Location of Testing Outlook (Revenue, USD Million, 2018 - 2030)
-
Laboratory Testing
-
Commercial/Private labs
-
Public Health Labs
-
-
Point of Care Testing
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
India
-
China
-
Japan
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Argentina
-
Brazil
-
-
Middle East & Africa
-
Saudi Arabia
-
UAE
-
South Africa
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global STD diagnostics market size was estimated at USD 10.62 billion in 2024 and is expected to reach USD 11.39 million in 2025.
b. The global STD diagnostics market is expected to witness a compound annual growth rate of 7.14% from 2024 to 2030 to reach USD 16.08 million in 2030.
b. Based on product, the consumables segment held the largest share of 70.01% in 2024, owing to the increased demand for consumables, increasing product approvals, and the introduction of innovative solutions.
b. Some key players operating in the STD diagnostics market BD; F. Hoffmann-La Roche Ltd.; Hologic, Inc.; bioMérieux SA; Abbott; Cepheid (Danaher Corporation); Qiagen; Seegene Inc; Thermo Fisher Scientific Inc.; DiaSorin S.p.A; OraSure Technologies Inc.; Bio-Rad Laboratories, Inc.
b. Key factors driving the STD diagnostics market growth include rising incidence of STDs, growing government policies to promote early diagnostics, increasing patient awareness, and technological advancement in STD diagnostics.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."




