- Home
- »
- Medical Devices
- »
-
Deep Brain Stimulation In Parkinson’s Disease Market Report, 2030GVR Report cover
![Deep Brain Stimulation In Parkinson’s Disease Market Size, Share & Trends Report]()
Deep Brain Stimulation In Parkinson’s Disease Market (2025 - 2030) Size, Share & Trends Analysis Report By Product (Single-channel, Dual-channel), By End-use (Hospitals, ASCs), By Region, And Segment Forecasts
- Report ID: GVR-4-68038-749-0
- Number of Report Pages: 100
- Format: PDF
- Historical Range: 2018 - 2023
- Forecast Period: 2025 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Market Size & Trends
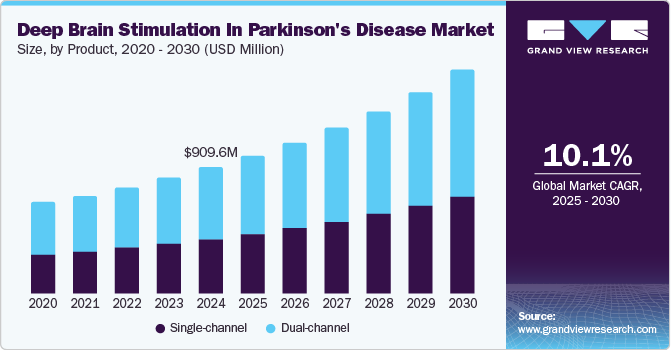
The global deep brain stimulation in Parkinson’s disease market size was valued at USD 909.6 million in 2024 and is projected to grow at a CAGR of 10.1% from 2025 to 2030. This growth is driven by the increasing prevalence of Parkinson's disease and other neurological disorders that necessitate advanced treatment options such as deep brain stimulation (DBS). Additionally, the rising geriatric population, which is more susceptible to Parkinson's disease, contributes significantly to the market growth. Technological advancements in DBS devices, such as improved microelectrode designs and robot-assisted implantation, are enhancing the effectiveness and safety of the treatment.
Government policies and laws play a crucial role in supporting the deep brain stimulation (DBS) market for Parkinson's disease through various initiatives aimed at enhancing research, funding, and access to treatment. Increased funding for research by government agencies, such as the National Institute of Neurological Disorders and Stroke (NINDS), facilitates clinical trials that assess the safety and efficacy of DBS devices, thereby encouraging innovation in this field. Additionally, favorable reimbursement policies by programs such as Medicare and the National Health Service (NHS) improve patient access to DBS therapy, making it more financially viable for healthcare providers to offer these advanced treatments.
Moreover, regulatory reforms aimed at streamlining the approval process for medical devices can significantly reduce barriers to market entry, allowing new DBS technologies to reach patients more quickly. For instance, the FDA has introduced pathways such as Early Feasibility Studies to support clinical investigations in smaller populations, which is particularly beneficial for rare disease treatments.
Product Insights
The dual-channel segment accounted for the largest share of 56.9% in 2024 due to dual-channel’s superior effectiveness in reducing patient tremors and enhancing the overall quality of life. Dual-channel DBS devices offer greater control and precision in delivering electrical stimulation to the targeted brain regions, making them highly preferred by neurosurgeons and patients alike. The robust performance and reliability of dual-channel systems have cemented their dominant position in the market.
The single-channel segment is expected to grow at a CAGR of 10.3% from 2025 to 2030, owing to the increasing demand for minimally invasive treatments and advancements in single-channel DBS technology. Single-channel devices are becoming more sophisticated, offering improved outcomes with fewer complications2. The segment's growth is also driven by the rising prevalence of Parkinson's disease and the need for cost-effective treatment options.
End-use Insights
The hospital segment dominated the market in 2024. Hospitals are often equipped with advanced medical technology and specialized personnel, enabling them to provide comprehensive care for patients requiring DBS therapy. The complexity of the procedure and the need for continuous monitoring and support throughout the treatment process make hospitals the preferred setting for many patients. Moreover, hospitals typically offer a multidisciplinary approach, combining neurologists, neurosurgeons, and other healthcare professionals, which enhances patient outcomes through coordinated care. As Parkinson’s disease often presents with a range of symptoms requiring ongoing management, hospitals are well-positioned to provide the integrated care necessary for effective treatment.
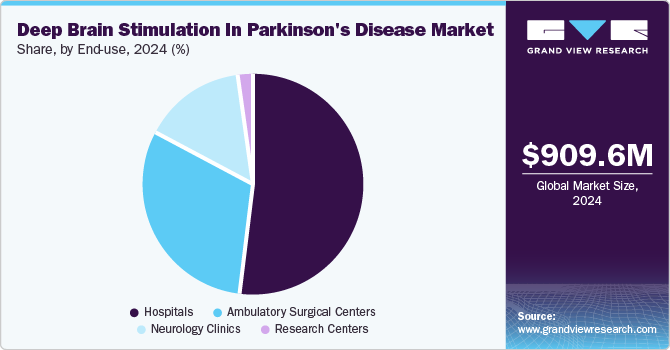
The Ambulatory Surgical Centers(ASCs) segment is projected to grow the fastest over the forecast period. ASCs provide a less complex and more cost-effective alternative to hospitals for surgical procedures, including DBS. Patients increasingly seek outpatient options that allow for quicker recovery times and lower overall healthcare costs. ASCs are often more efficient in terms of scheduling and can offer a streamlined experience, minimizing wait times and enhancing patient satisfaction. Furthermore, advancements in minimally invasive techniques and technologies in the field of neuromodulation are making it possible for procedures such as DBS to be performed safely in an outpatient setting. This shift not only improves accessibility for patients but also aligns with the growing trend of outpatient care across various medical specialties.
Regional Insights
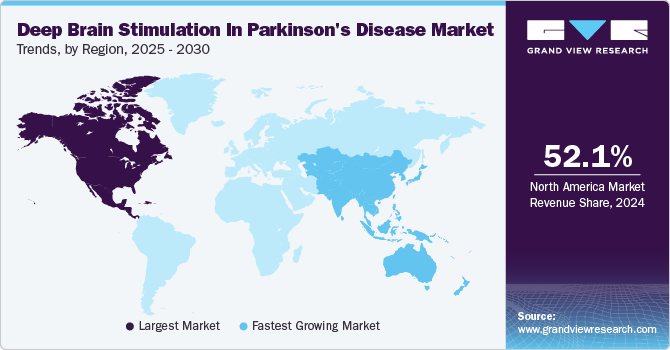
North America DBS in the Parkinson’s disease market dominated the largest revenue share in 2024. The region's dominance can be attributed to several factors, including the high prevalence of Parkinson’s disease, advanced healthcare infrastructure, and significant government and private funding for research and development. Technological advancements, such as robot-assisted implantation and enhanced microelectrode designs, have further propelled the market growth in this region.
U.S. DBS In Parkinson’s Disease Market Trends
The U.S. DBS in the Parkinson’s disease market accounted for 88.1% of revenue of the North American market in 2024 driven by factors such as the increasing prevalence of Parkinson’s disease, technological advancements in DBS devices, and substantial government funding for research and development. The National Institute of Neurological Disorders and Stroke (NINDS) has been a significant contributor, providing substantial financial support for PD research.
Europe Deep Brain Stimulation In Parkinson’s Disease Market Trends
Europe's DBS in Parkinson’s disease market is projected to grow at a CAGR of 10.5% from 2025 to 2030 fueled by increasing awareness of DBS therapy, rising prevalence of Parkinson’s disease, and supportive government policies. The region is witnessing a surge in demand for advanced DBS devices, driven by the aging population and the need for effective treatment options for neurological disorders1. European countries are also investing in research and development to improve DBS technologies and make them more accessible to patients.
Asia Pacific Deep Brain Stimulation In Parkinson’s Disease Market Trends
The Asia Pacific DBS in Parkinson’s disease market is anticipated to grow fastest throughout the forecast period. This rapid growth can be attributed to the increasing prevalence of Parkinson’s disease, rising healthcare expenditure, and growing awareness about DBS therapy. The region is also benefiting from technological advancements and the introduction of innovative DBS devices. Governments in the Asia Pacific region are actively supporting the adoption of DBS therapy through favorable policies and funding for research and development.
Key Deep Brain Stimulation In Parkinson’s Disease Company Insights
Some of the key companies in the deep brain stimulation In Parkinson’s disease market include ABBOTT, Medtronic, Boston Scientific Corporation, and others.
-
Medtronic plc has been a pioneer in the deep brain stimulation (DBS) market, being the first company to receive FDA approval for a DBS device for Parkinson's disease back in 1997. Its Activa line of devices includes both rechargeable and non-rechargeable batteries, catering to different patient needs. In 2020, Medtronic launched the Percept device, which stands out as the first DBS system capable of sensing and recording brain signals, allowing for more precise adjustments based on individual patient responses. Recently, Medtronic received FDA approval for Asleep DBS surgery for Parkinson's and essential tremor patients.
-
Abbott is a prominent player in the deep brain stimulation (DBS) market for Parkinson's disease, particularly known for its Infinity DBS system. This system is unique as it is the first directional DBS device approved for all major targets used in treating movement disorders, including the internal globus pallidus (GPi), which is crucial for managing Parkinson's symptoms. Abbott's Infinity system utilizes advanced technology, including an iOS software platform, allowing clinicians to program the device using an iPad mini, enhancing efficiency in patient management.
Key Deep Brain Stimulation In Parkinson’s Disease Companies:
The following are the leading companies in the deep brain stimulation in Parkinson’s disease market. These companies collectively hold the largest market share and dictate industry trends.
- ABBOTT
- Medtronic
- Boston Scientific Corporation
Recent Developments
-
In August 2024, Medtronic plc received FDA approval for Asleep DBS surgery for Parkinson's and essential tremor patients. This innovative approach allows patients to undergo DBS surgery either awake or asleep, providing greater flexibility and comfort.
Deep Brain Stimulation In Parkinson’s Disease Market Report Scope
Report Attribute
Details
Market size value in 2025
USD 993.6 million
Revenue forecast in 2030
USD 1.61 billion
Growth Rate
CAGR of 10.1% from 2024 to 2030
Base year for estimation
2024
Historical data
2018 - 2023
Forecast period
2025 - 2030
Quantitative units
Revenue in USD million/billion, and CAGR from 2025 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Product, end-use, region
Regional scope
North America, Europe, Asia Pacific, Latin America, MEA
Country scope
U.S., Canada, Mexico, Germany, UK, France, Italy, Spain, Sweden, Norway, Denmark, China, Japan, India, Indonesia, South Korea, Thailand, Australia, Philippines, Malaysia, Singapore, Brazil, Argentina, Colombia, Chile, Venezuela, South Africa, Saudi Arabia, UAE, Turkey, Iran, Kuwait
Key companies profiled
ABBOTT, Medtronic, Boston Scientific Corporation
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Deep Brain Stimulation In Parkinson’s Disease Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global deep brain stimulation in Parkinson’s disease market report based on product, end-use, and region.
-
Product Outlook (Revenue, USD Million, 2018 - 2030)
-
Single-channel
-
Dual-channel
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Neurology Clinics
-
Ambulatory Surgical Centers
-
Research Centers
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Indonesia
-
South Korea
-
Thailand
-
Australia
-
Philippines
-
Malaysia
-
Singapore
-
-
Latin America
-
Brazil
-
Argentina
-
Colombia
-
Chile
-
Venezuela
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
United Arab Emirates
-
Turkey
-
Iran
-
Kuwait
-
-
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










