
De-identified Health Data Market Size, Share & Trends Analysis Report By Type Of Data, By Application, By End-use (Pharmaceutical Companies, Biotechnology Firms, Medical Device Manufacturers, Healthcare Providers), By Region, And Segment Forecasts, 2025 - 2030
- Report ID: GVR-4-68040-481-1
- Number of Report Pages: 120
- Format: PDF
- Historical Range: 2018 - 2024
- Forecast Period: 2025 - 2030
- Industry: Healthcare
De-identified Health Data Market Size & Trends
The global de-identified health data market size was estimated at USD 8.09 billion in 2024 and is expected to grow at a CAGR of 9.07% from 2025 to 2030. The market growth is driven by the increasing integration of data analytics in healthcare, which supports large-scale studies and predictive modeling without breaching patient confidentiality. Regulatory frameworks such as GDPR and HIPAA further incentivize using de-identified data for compliance. Moreover, AI and machine learning advancements amplify the need for extensive privacy-compliant datasets to improve diagnostic and therapeutic methods. In addition, the surge in data from wearable devices, sensors, and electronic health records (EHRs) has expanded the scope for de-identified data in secondary applications.
De-identified health data is essential for clinical research as it allows researchers to analyze large datasets while protecting patient privacy. This data identifies trends, evaluates treatment effectiveness, and supports population health studies without compromising individual identities. By leveraging de-identified data, researchers can enhance the quality of their findings and facilitate advancements in medical knowledge and practice.
For instance, in April 2023, Philips and MIT's Institute for Medical Engineering and Science (IMES) collaborated to develop an enhanced critical care dataset to advance clinical research and AI applications in healthcare. This dataset includes de-identified data from ICU patients and integrates comprehensive clinical information to support researchers and educators in gaining insights into critical care and improving patient outcomes. The initiative fosters innovation in AI-driven healthcare solutions, contributing to more accurate diagnostics and personalized treatments.
De-identification facilitates collaboration and innovation within the healthcare sector by enabling secure patient data sharing across various healthcare systems, thereby advancing diagnostic and treatment technologies. Moreover, it provides critical data necessary for training AI systems, enhancing the accuracy and relevance of medical imaging for disease detection and analysis. This approach protects patient privacy and drives improvements in healthcare outcomes.
For instance, in December 2023, nference, a software company focused on transforming healthcare data for research, partnered with Emory Healthcare, Georgia's largest academic health system, to enhance access to diverse, aggregated, de-identified data. This initiative aims to accelerate research efforts, improve disease diagnosis, and facilitate the development of new treatments. The collaboration reflects a mutual commitment to advancing medical knowledge, promoting innovation, and enhancing the health and well-being of individuals and communities globally.
“This collaboration with nference allows us to join a federated data network of leading institutions that will enable ground-breaking research. Together, we can work to improve lives and provide hope, tackling some of the most critical health care challenges of our time while delivering comprehensive, data-driven insights.”
-Joe Depa, chief data and analytics officer at Emory Healthcare and Emory University
Industry Dynamics
The degree of innovation in the de-identified health data industry is high. Innovation in the de-identified health data industry is driven by advancements in data analytics, artificial intelligence (AI), and machine learning (ML), which enhance the extraction of insights while preserving patient privacy. For instance, in August 2020, the Defense Innovation Unit employed de-identified health data to train AI models for early cancer detection. By utilizing anonymized patient data, the initiative aims to enhance the accuracy of AI algorithms while ensuring patient privacy. This approach supports the development of advanced diagnostic tools that could significantly improve cancer outcomes through timely and precise disease identification.
The M&A activities, such as mergers, acquisitions, and partnerships, enable companies to expand geographically, financially, and technologically. For instance,in June 2021, Datavant and Ciox Health announced their merger, creating the largest neutral and secure health data ecosystem in the U.S. This merger aims to enhance the interoperability of healthcare data, facilitating the secure sharing of de-identified patient datasets across various healthcare entities. The combined entity will focus on advancing healthcare insights and improving patient outcomes while maintaining compliance with privacy regulations.
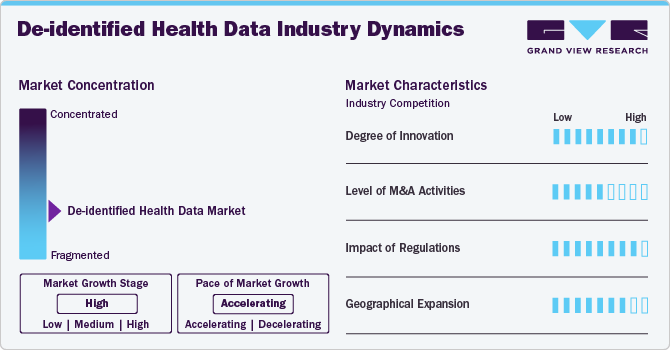
Regulations governing the de-identified health data market focus on ensuring patient privacy and data security. Key frameworks include the Health Insurance Portability and Accountability Act (HIPAA), which establishes guidelines for de-identifying health data to prevent patient identification. In addition, Europe's General Data Protection Regulation (GDPR) imposes strict requirements on data handling and consent, influencing global practices. Organizations must adhere to these regulations while leveraging de-identified data for research, analytics, and other applications to ensure compliance and protect individual privacy.
Geographic expansion significantly drives the market growth by increasing market penetration and revenue, enabling access to diverse data sources, and fostering regulatory compliance and standardization. For instance, in April 2018, F. Hoffmann-La Roche acquired Flatiron Health for approximately USD 1.9 billion, enhancing its capabilities in oncology-focused EHR software and real-world evidence. Flatiron Health, based in the U.S., provides de-identified health data solutions. Hence, this acquisition strengthened Roche's industry position by allowing it to expand its geographic presence and enhance its offerings in the de-identified health data industry.
Type Of Data Insights
The clinical data segment dominated with the largest share of approximately 17.00% in 2024. The segment's dominance is attributed to its crucial role in research, treatment development, and patient care optimization. The extensive availability of clinical data enables the identification of treatment outcomes and patient demographics, which are essential for advancing personalized medicine. For instance, in March 2024, Tempus announced the contribution of de-identified tumor profiles' data, including limited clinical information from over 3,000 cancer diagnoses to the National Cancer Institute (NCI). This marks a unique addition to NCI's planned Data Enclave and aims to support the advancement of cancer research by enhancing insights from individual cancer cases. This initiative aligns with NCI's mission to improve cancer outcomes through data-driven research.
The epidemiological data segment is expected to grow at the fastest CAGR during the forecast period. The growth is attributed to the increasing public health initiatives and a heightened focus on disease prevention. The demand for data to track disease patterns, identify risk factors, and inform health policies drives this trend. Moreover, advancements in data analytics and technology facilitate the effective use of large datasets for epidemiological research, further driving market growth.
Application Insights
Clinical research and trials dominated the application segment with the largest revenue share in 2024. The segment’s largest share is attributed to its key role in advancing treatment methods and ensuring patient safety. These factors collectively increase the demand for clinical trials, which depend highly on de-identified data, thereby driving market growth. For instance, as of September 2024, ClinicalTrials.gov had 61,624 registered studies with posted results over time.
Percentage of recruiting studies by location (as of September 2024)
|
Location |
Number of Recruiting Studies and Percentage of Total |
|
U.S. only |
20,384 (30%) |
|
Non-U.S. only |
44,267 (65%) |
|
Both U.S. and non-U.S. |
3,059 (5%) |
|
Not provided |
33 (0%) |
The drug discovery and development segment is expected to grow at the fastest CAGR during the forecast period. The growth is attributed to the increasing reliance on real-world evidence (RWE) to accelerate drug development. De-identified data enables researchers to analyze diverse patient populations, predict drug responses, and identify potential safety concerns early in the development process. For instance, in July 2023, nference and Vanderbilt University Medical Center (VUMC) entered into an agreement to enhance real-world evidence (RWE) generation for complex diseases. The collaboration integrates VUMC’s extensive longitudinal, multi-modal data with nference’s AI-driven federated platform. This partnership aims to advance scientific insights, benefiting drug discovery and patient care by leveraging real-world data for more effective healthcare solutions.
End-use Insights
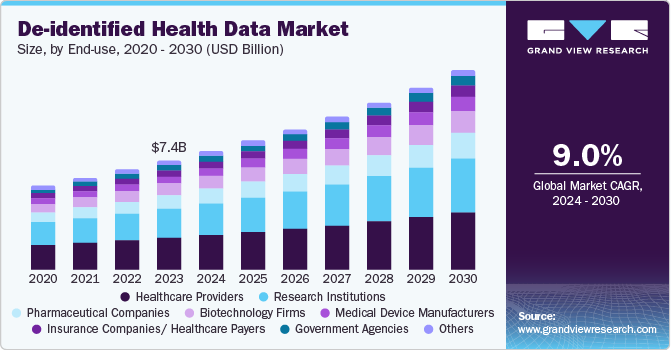
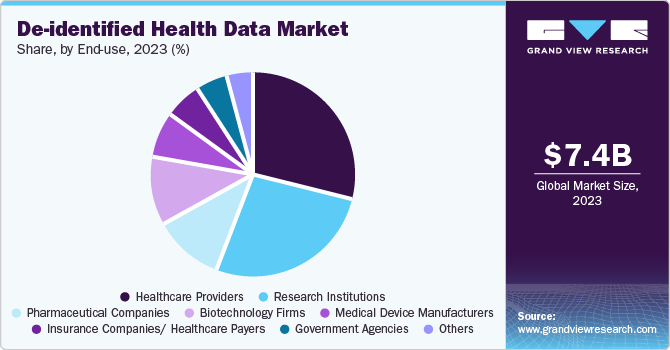
Healthcare providers dominated the end use segment in the market, with the largest revenue share in 2024. The healthcare providers segment leads the de-identified health data market owing to its crucial role in clinical decision-making, treatment optimization, and patient outcome improvement. Providers rely on de-identified data for research, population health management, and quality improvement initiatives, enabling them to analyze trends without breaching patient privacy. In addition, regulatory requirements for data privacy and the need for evidence-based care further drive the demand for de-identified data to enhance operational efficiency and support clinical advancements.
The pharmaceutical companies segment is expected to grow at the fastest CAGR over the forecast period. The growth is attributed to its essential role in drug development, clinical trials, and precision medicine. Pharmaceutical firms increasingly rely on de-identified data to analyze patient populations, assess drug safety and efficacy, and optimize trial designs without compromising privacy. Hence, market players undertake several strategic initiatives to leverage the advantage of de-identified health datasets. For instance, in July 2024, QuantHealth partnered with OMNY Health to leverage OMNY’s vast de-identified health data network. This collaboration aims to enhance clinical trial design, evidence-based practices, and medical research.
Regional Insights
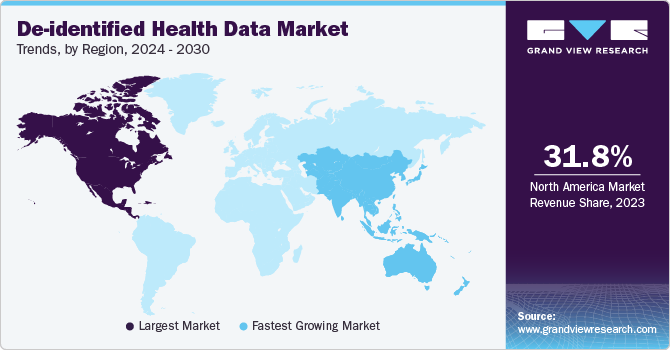
North America de-identified health data market dominated with a revenue share of over 31.53% in 2024. The region has an advanced healthcare infrastructure and significant technological investment, particularly in data analytics and AI. Moreover, stringent regulatory frameworks such as HIPAA enhance the focus on data privacy, encouraging the use of de-identified data for research while ensuring compliance. Furthermore, the presence of leading pharmaceutical and biotech companies accelerates the demand for high-quality data to support clinical trials and drug development. The strong emphasis on innovation and research further strengthens North America's leading position in this market.
U.S. De-identified Health Data Market Trends
De-identified health data industry in the U.S. is driven by its extensive healthcare system and significant investment in health information technology. For instance, in June 2024, the U.S. Department of Health and Human Services awarded USD 56 million to enhance health centers' technology for improved care quality. The funding supports the modernization of UDS reporting, allowing for streamlined processes and reduced time spent on chart audits. The initiative aligns with Health Level 7 (HL7) FHIR API standards, facilitating efficient health data exchange. All data collected through this initiative will be de-identified and secured, with compliance required with the HHS Safe Harbor Method for patient data de-identification to adhere to HIPAA regulations.
Europe De-identified Health Data Market Trends
De-identified health data industry in Europe is expected to be driven by its stringent regulatory framework, which emphasizes data privacy and protection, such as the GDPR. This regulatory environment fosters a culture of data sharing while ensuring compliance with privacy standards. Furthermore, advanced healthcare systems and extensive research institutions enhance the demand for de-identified data to support clinical studies and public health initiatives. The growing focus on personalized medicine and digital health solutions further drives the adoption of de-identified data across the region.
De-identified health data market in the UK is expected to be driven by significant government initiatives to advance health data research. For instance, in July 2024, the UK government secured nearly USD 55.78 million (EUR 50 million) to support the UK Biobank, a leading health research resource, following new backing from the pharmaceutical industry. This funding will enhance the biobank's capabilities in storing and analyzing de-identified health data, facilitating advancements in medical research. The initiative aims to facilitate innovations in treatment and disease prevention, strengthening UK's position in health data research.
Germany de-identified health data market held a significant market share in 2024. This is owing to its advanced healthcare system and commitment to data protection regulations, particularly under GDPR. The country's advanced research infrastructure and numerous healthcare institutions facilitate collecting and utilizing de-identified data for clinical studies. Moreover, Germany's emphasis on innovation in healthcare technology supports integrating de-identified data in research and public health initiatives.
Asia Pacific De-identified Health Data Market Trends
De-identified health data industry in Asia Pacific is expected to grow at the fastest CAGR during the forecast period. The growth is attributed to rapid advancements in healthcare infrastructure and technology. Increasing investments in health IT and data analytics and a growing demand for personalized medicine are driving this growth. Moreover, rising awareness of data privacy regulations pushes organizations to adopt de-identified data solutions for compliance. The region's expanding pharmaceutical and biotech sectors further contribute to the demand for comprehensive health data for research and clinical applications.
De-identified health data market in Japan held a significant market share in 2024. The market share is attributed to several initiatives market players undertake to advance Japan’s healthcare. For instance, in June 2024, SoftBank Group formed a joint venture named "SB TEMPUS" with Tempus to enhance healthcare in Japan by applying medical data and AI. The venture aims to offer precision medicine services by leveraging Tempus's extensive expertise and technology, including one of the industry's largest collections of de-identified molecular, clinical, and imaging data. Tempus's connections to approximately 50% of U.S. oncologists will support the initiative's objectives.
India de-identified health data market is expected to be driven by the growing awareness and use of de-identified data solutions. For instance, in July 2024, Miimansa AI published research highlighting innovative methods for de-identifying clinical discharge summaries in India using Large Language Models (LLMs). This study responds to the growing demand for effective data de-identification techniques amid increasing digitization in healthcare. The research enhances de-identification efficacy by employing LLMs to create synthetic clinical reports while safeguarding patient privacy and maintaining data utility.
Key De-identified Health Data Company Insights
The growing number of collaborations, partnerships, and mergers & acquisitions among industry players are enabling them to gain a competitive edge in the market. For instance, in September 2024, ICON plc partnered with IBM and announced advancements in clinical trial processes through de-identified health data, which enhances patient recruitment and optimizes study design. The initiative aims to improve trial efficiency and accelerate drug development by leveraging vast datasets while ensuring patient privacy. The emphasis on de-identified data enables researchers to gain insights without compromising individual privacy, thereby transforming clinical trial methodologies.
Key De-identified Health Data Companies:
The following are the leading companies in the de-identified health data market. These companies collectively hold the largest market share and dictate industry trends.
- IQVIA
- Oracle (Cerner Corporation)
- Merative (Truven Health Analytics)
- Optum, Inc. (UnitedHealth Group)"
- ICON plc
- Veradigm LLC (Formerly known as Allscripts)
- IBM
- Flatiron Health (F. Hoffmann-La Roche Ltd)
- Premier, Inc.
- Shaip
- Komodo Health, Inc.
- Evidation Health, Inc.
- Medidata
- Clarify Health Solutions
- Satori Cyber Ltd.
Recent Developments
-
In September 2024, ICON announced a collaboration with Intel to utilize de-identified data from its clinical research platform alongside Intel's AI technology. This partnership enhances patient recruitment and streamlines clinical trial processes by deriving insights from de-identified patient data. The initiative aims to advance precision medicine and improve efficiencies in drug development and outcomes by integrating ICON's clinical trial expertise with Intel's AI capabilities.
-
In February 2024, Veradigm published its first Veradigm Insights Report: Cardiovascular Conditions in 2024, analyzing de-identified real-world data from 53 million cardiovascular patients. The report assesses the prevalence of cardiovascular disease (CVD) and related conditions across all U.S. states, with demographic breakdowns based on age, ethnicity, and sex.
-
In July 2021, Verana Health and Komodo Health partnered to integrate Komodo’s Healthcare Map into Verana’s de-identified EHR datasets, spanning over 325 million patient journeys. This collaboration aims to provide life sciences researchers with detailed insights into patient pathways, encompassing treatment histories, hospitalizations, and socioeconomic factors. The partnership is expected to enhance research efforts in ophthalmology, neurology, and urology by combining clinical outcomes with real-world patient data, supporting more informed treatment development.
De-identified Health Data Market Report Scope
|
Report Attribute |
Details |
|
Market size value in 2025 |
USD 8.80 billion |
|
Revenue forecast in 2030 |
USD 13.59 billion |
|
Growth rate |
CAGR of 9.07% from 2025 to 2030 |
|
Actual data |
2018 - 2024 |
|
Forecast period |
2025 - 2030 |
|
Quantitative units |
Revenue in USD million/billion and CAGR from 2025 to 2030 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Type of data, application, end-use, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
|
Country scope |
U.S.; Canada; Mexico; UK; Germany; Spain; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait |
|
Key companies profiled |
IQVIA; Oracle (Cerner Corporation); Merative (Truven Health Analytics); Optum, Inc. (UnitedHealth Group); ICON plc; Veradigm LLC (Formerly known as Allscripts); IBM; Flatiron Health (F. Hoffmann-La Roche Ltd); Premier, Inc.; Shaip; Komodo Health, Inc.; Evidation Health, Inc.; Medidata; Clarify Health Solutions; Satori Cyber Ltd. |
|
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. Explore purchase options |
Global De-identified Health Data Market Report Segmentation
This report forecasts revenue growth and provides at global, regional, and country levels an analysis of the latest trends in each of the sub-segments from 2018 - 2030. For this report, Grand View Research has segmented the global de-identified health data market report based on type of data, application, end-use, and region:
-
Type of Data Outlook (Revenue, USD Million, 2018 - 2030)
-
Clinical Data
-
Genomic Data
-
Patient Demographics
-
Prescription Data
-
Claims Data
-
Behavioral Data
-
Wearable and Sensor Data
-
Survey and Patient-Reported Data
-
Imaging Data
-
Laboratory Data
-
Hospital and Provider Data
-
Social Determinants of Health (SDoH) Data
-
Pharmacogenomic Data
-
Biometric Data
-
Operational and Financial Data
-
Epidemiological Data
-
Healthcare Utilization Data
-
Others
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
Clinical Research and Trials
-
Public Health
-
Precision Medicine
-
Health Economics and Outcomes Research (HEOR)
-
Population Health Management
-
Drug Discovery and Development
-
Healthcare Quality Improvement
-
Insurance Underwriting and Risk Assessment
-
Market Access and Commercial Strategy
-
Business Intelligence and Operational Efficiency
-
Telemedicine and Remote Monitoring
-
Patient Engagement and Support Programs
-
Others
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Pharmaceutical Companies
-
Biotechnology Firms
-
Medical Device Manufacturers
-
Healthcare Providers
-
Insurance Companies/ Healthcare Payers
-
Research Institutions
-
Government Agencies
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global de-identified health data market size was estimated at USD 8.09 billion in 2024 and is expected to reach USD 8.80 billion in 2025.
b. The global de-identified health data market is expected to grow at a compound annual growth rate of 9.07% from 2025 to 2030 to reach USD 13.59 billion by 2030.
b. North America dominated the global de-identified health data market with a share of 31.53% in 2024. This is attributable to the presence of leading pharmaceutical and biotech companies, advanced healthcare infrastructure and significant technological investment, particularly in data analytics and AI. In addition, stringent regulatory frameworks such as HIPAA enhance the focus on data privacy, encouraging the use of de-identified data for research while ensuring compliance.
b. Some key players operating in the global de-identified health data market include IQVIA; Oracle (Cerner Corporation); Merative (Truven Health Analytics); Optum, Inc. (UnitedHealth Group); ICON plc; Veradigm LLC (Formerly known as Allscripts); IBM; Flatiron Health (F. Hoffmann-La Roche Ltd); Premier, Inc.; Shaip; Komodo Health, Inc.; Evidation Health, Inc.; Medidata; Clarify Health Solutions; and Satori Cyber Ltd.
b. Key factors that are driving the market growth include rising demand for healthcare data, growth in AI and Machine Learning, growing adoption of healthcare analytics (data-driven decision-making), and growth in Real-World Data (RWD) and Real-World Evidence (RWE).
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."




