
Cervical Cancer Treatment Market Size, Share & Trends Analysis Report By Type (Squamous Cell Carcinoma), By Treatment, By End-use (Hospital & Clinics, Ambulatory Surgery Centers), By Region, And Segment Forecasts, 2024 - 2030
- Report ID: GVR-2-68038-718-6
- Number of Report Pages: 100
- Format: PDF, Horizon Databook
- Historical Range: 2018 - 2022
- Forecast Period: 2024 - 2030
- Industry: Healthcare
Cervical Cancer Treatment Market Trends
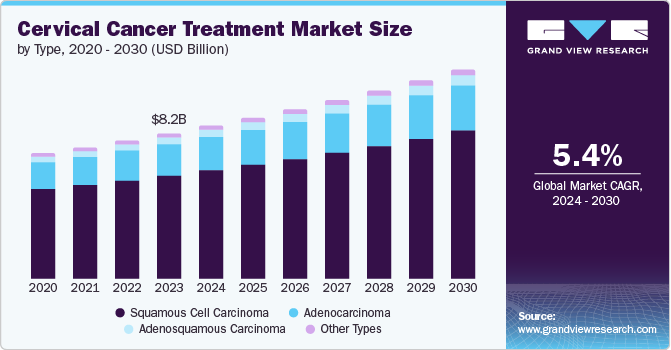
The global cervical cancer treatment market size was valued at USD 8.16 billion in 2023 and is projected to grow at a CAGR of 5.4% from 2024 to 2030. This growth is attributed to advancements in oncology technology, increasing incidence of cervical cancer, recent advancements in imaging devices brought about by leading market players, and general growth in women’s health consciousness and changes in their lifestyle. Furthermore, women living with HIV are significantly more susceptible, highlighting the intersection of health disparities and cervical cancer risk.
In addition, cervical cancer's global growth is limited access to HPV vaccination, screening, and treatment, particularly in low- and middle-income countries where the burden is highest. Socioeconomic determinants, such as poverty and gender biases, exacerbate these inequities.
Rising cases of cervical cancer are a significant factor in the growth of the market. According to the latest WHO data, there were recorded 660,000 new cases of cervical cancer and 350,000 deaths in 2022. 94% of these deaths have been caused in low and middle-income countries. Sub-Saharan Africa, South-East Asia, and Central America have the highest rates of cervical cancer. The leading cause of this disease is the Human papillomavirus (HPV), which has caused 95% of cervical deaths. These statistics show the seriousness of the issue of cervical cancer and draw the attention of the world for creating the solution and creating an opportunity for the growth of the market.
Moreover, in 2024, the Indian government, under the ‘NARI SHAKTI’ scheme, announced vaccination for girls aged 9 to 14 to prevent cervical cancer. These UN, WHO, and other major economic country government initiatives are increasing the opportunity for the industry players to increase their market share and thus grow the market significantly.
Type Insights
Squamous cell carcinoma type dominated the market and accounted for the largest revenue share of 71.1% in 2023. This growth is attributed to persistent infection with high-risk human papillomavirus (HPV) subtypes, mainly HPV 16 and 18, which account for most cases. In addition, limited access to HPV vaccination, screening, and treatment in low- and middle-income countries exacerbates the burden. Furthermore, socioeconomic determinants, such as poverty and gender biases, further contribute to the inequities in cervical cancer prevention and control.
The adenocarcinoma type segment is expected to grow at a CAGR of 5.8% over the forecast years owing to the growing access to HPV vaccine in low- and middle-income countries to prevent this type of cancer. In addition, increasing incidence rates of cervical adenocarcinoma, driven by persistent high-risk HPV infections, are a primary concern. Limited access to effective screening and vaccination programs exacerbates the issue, particularly in low- and middle-income countries. Furthermore, rising awareness of cervical cancer among women and government initiatives aimed at improving healthcare access contribute to market growth.
Treatment Insights
Chemotherapy treatment led the market and accounted for the largest revenue share of 33.9% in 2023. This growth is attributed to the advancements in drug development that have significantly enhanced treatment options for cervical adenocarcinoma. In addition, increased investments in healthcare organizations have improved overall treatment procedures and accessibility. Furthermore, there is a rising trend toward personalized treatment approaches, focusing on tailored medicinal strategies for patients.
The immunotherapy treatment segment is expected to grow at a CAGR of 10.4% over the projected years. This growth is driven by rising investments in R&D, leading to technological advancements in treatment, enhanced therapeutic strategies to improve patients' outcomes, and minimizing adverse effects. The market is growing significantly with increasing awareness and campaigns about the treatment.
End-use Insights
Hospitals & clinics segment dominated the market and accounted for the largest revenue share of 65.0% in 2023. This growth is attributed to the enhanced delivery procedures in hospitals & clinics with offerings such as targeted therapy, radiation therapy, immunotherapy, minimally invasive surgeries, and many other methods. Furthermore, government initiatives and healthcare policies to improve the quality of treatment in hospitals & clinics are also driving the market significantly.
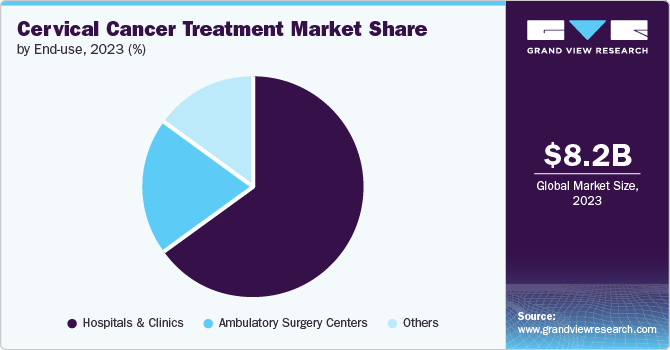
Ambulatory surgery centers are anticipated to witness the fastest growth, with a CAGR of 5.8% over the forecast years. This growth is attributed to expanding insurance coverage, which enables patients to receive treatments in these centers, and stringent regulatory regulations to improve the quality of these centers, which ensures patients receive safe therapies. These factors change patient preferences and increase the treatment at these centers, leading to the significant growth of this segment.
Regional Insights
The North America cervical cancer treatment market dominated the global market and accounted for the largest revenue share of 39.4% in 2023. This growth is attributed to the partnership between government bodies and healthcare organizations to increase awareness of cervical cancer treatment and enhanced reimbursement policies to provide better services, encouraging patients to avail of the treatment.
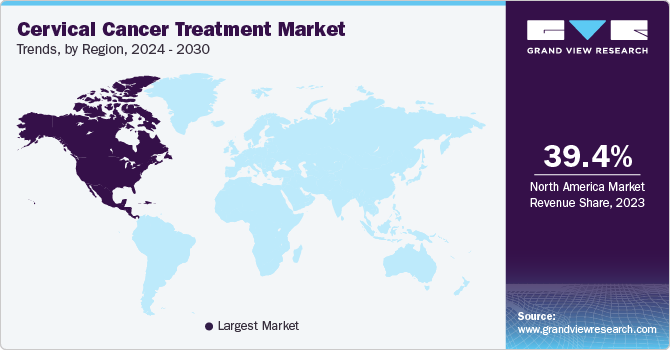
U.S. Cervical Cancer Treatment Market Trends
The cervical cancer treatment market in the U.S. dominated the North American market with the largest revenue share of 75.5% in 2023, owing to increasing cases of cervical cancer. This leads to increasing government support for solving the issue, which increases the opportunity for companies to grow in the country. Technological advancements that have enhanced treatment procedures encourage patients to avail themselves of treatment, thus increasing the market significantly in the region.
Canada cervical cancer treatment market is expected to grow at a CAGR of 11.3% over the projected years. This growth is driven by rising incidence rates, government support, technological innovation in treatment methods, enhancement in public awareness of prevention, and demographic changes inclining towards an aging population. Furthermore, advancements in diagnostic technologies and treatment options enhance patient outcomes, while collaborative efforts between healthcare providers and organizations support effective management and prevention strategies.
Europe Cervical Cancer Treatment Market Trends
The cervical cancer treatment market in Europe is expected to grow significantly owing to partnerships between pharmaceutical companies, healthcare providers, and research institutions, which have led to innovations, technological advancements, and improved treatment regulations. Furthermore, the active participation of NGOs in raising awareness about cervical cancer and growing the market in the region is significant.
Asia Pacific Cervical Cancer Treatment Market Trends
The cervical cancer treatment market in Asia Pacific is expected to grow at a CAGR of 6.0% over the forecast years. This growth is attributed to factors such as the growing incidence of cervical cancer, enhanced knowledge through education activities, government support measures, advanced healthcare systems, increased HPV vaccine coverage, and economic development.
China cervical cancer treatment market held a substantial market share in 2023 owing to the increased rate of cervical cancer, government support, growing awareness among women, improved technology, increased accessibility to healthcare, increased healthcare costs, and protected human papillomavirus vaccination programs.
The UK cervical cancer treatment market is expected to grow rapidly in the coming years due to the rising importance of personalized medicine, increasing recognition of women's health issues, and increasing government funding playing a vital role in supporting cervical cancer treatment are growing the market significantly.
Key Cervical Cancer Treatment Company Insights
Some key cervical cancer treatment market companies include Pfizer Inc., Merck & Co., Inc., Johnson & Johnson Services, Inc., and others. Organizations focus on increasing their customer base to gain a competitive edge in the industry. Therefore, key players are taking several strategic initiatives, such as mergers and acquisitions and partnerships with other major companies.
Key Cervical Cancer Treatment Companies:
The following are the leading companies in the cervical cancer treatment market. These companies collectively hold the largest market share and dictate industry trends.
- Merck & Co., Inc.
- Bristol-Myers Squibb Company
- F. Hoffmann-La Roche Ltd
- Pfizer Inc.
- Eli Lilly and Company.
- Novartis AG
- AstraZeneca
- GSK plc.
- AbbVie Inc.
- Johnson & Johnson Services, Inc.
Recent Developments
-
In April 2024, The FDA granted full sanction for TIVDAK (tisotumab vedotin-tftv) to treat recurrent or metastatic cervical cancer. TIVDAK, an antibody-drug conjugate (ADC), has become the first of its kind to show positive overall survival data in patients with recurrent or metastatic cervical cancer who have undergone prior treatment. This full approval by the FDA represents a significant advancement in managing this challenging disease, as TIVDAK has proven more effective than standard chemotherapy regimens. The approval is based on the global, randomized, Phase 3 innovaTV 301 clinical trial results.
-
In February 2024, BD partnered with Camtech Health to enhance cervical cancer screening in Singapore by introducing the first-ever at-home self-collection option for HPV testing. This initiative aims to improve screening rates, currently below 50%, by allowing women to collect samples privately. The program utilizes the HPV Test of Camtech Health and Onclarity HPV Assay of BD, which detects multiple high-risk HPV strains, facilitating better access and awareness in cervical cancer prevention efforts.
Cervical Cancer Treatment Market Report Scope
|
Report Attribute |
Details |
|
Market size value in 2024 |
USD 8.58 billion |
|
Revenue forecast in 2030 |
USD 11.74 billion |
|
Growth rate |
CAGR of 5.4% from 2024 to 2030 |
|
Base year for estimation |
2023 |
|
Historical data |
2018 - 2022 |
|
Forecast period |
2024 - 2030 |
|
Quantitative units |
Revenue in USD million/billion and CAGR from 2024 to 2030 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Type, treatment, end-use, region |
|
Regional scope |
North America, Europe, Asia Pacific, Latin America, MEA |
|
Country scope |
U.S., Canada, Mexico, Germany, UK, France, Italy, Spain, Denmark, Sweden, Norway, China, Japan, India, South Korea, Australia, Thailand, Brazil, Argentina, Saudi Arabia, UAE, Kuwait, South Africa |
|
Key companies profiled |
Merck & Co., Inc.; Bristol-Myers Squibb Company; F. Hoffmann-La Roche Ltd; Pfizer Inc.; Eli Lilly and Company.; Novartis AG; AstraZeneca; GSK plc.; AbbVie Inc.; Johnson & Johnson Services, Inc. |
|
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. Explore purchase options |
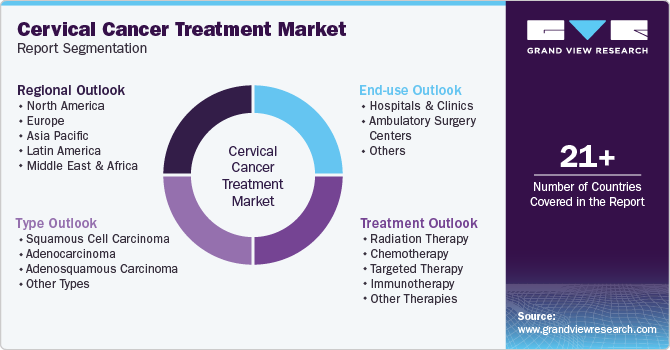
Global Cervical Cancer Treatment Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and analyzes the latest industry trends in each sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global cervical cancer treatment market report based on type, treatment, end use, and region:
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Squamous Cell Carcinoma
-
Adenocarcinoma
-
Adenosquamous Carcinoma
-
Other Types
-
-
Treatment Outlook (Revenue, USD Million, 2018 - 2030)
-
Radiation Therapy
-
External Beam Radiation Therapy (EBRT)
-
Brachytherapy
-
-
Chemotherapy
-
Cisplatin
-
Carboplatin
-
Paclitaxel
-
Other Chemotherapeutic Agents
-
-
Targeted Therapy
-
Bevacizumab (Avastin)
-
Other Targeted Therapies
-
-
Immunotherapy
-
Pembrolizumab (Keytruda)
-
Other Immune Checkpoint Inhibitors
-
-
Other Therapies
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals & Clinics
-
Ambulatory Surgery Centers
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
China
-
Japan
-
India
-
South Korea
-
Australia
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
Saudi Arabia
-
UAE
-
South Africa
-
Kuwait
-
-
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."




