
Capsule Endoscopy Market Size, Share & Trends Analysis Report By Type (Small Bowel Capsule Endoscopy, Esophageal Capsule Endoscopy), By Application (OGIB, Crohn’s Disease), By End-use (Hospitals, Outpatient Facilities), By Region, And Segment Forecasts, 2025 - 2030
- Report ID: 978-1-68038-439-0
- Number of Report Pages: 80
- Format: PDF
- Historical Range: 2018 - 2024
- Forecast Period: 2025 - 2030
- Industry: Healthcare
Capsule Endoscopy Market Size & Trends
The global capsule endoscopy market size was estimated at USD 443.8 million in 2024 and is expected to expand at a CAGR of 7.9% from 2025 to 2030. The increasing prevalence of Gastrointestinal (GI) diseases & colon cancer, rising demand for minimally invasive diagnostic procedures, and introduction of technologically advanced capsule endoscopes are the major factors driving the market. For instance, according to the Canadian Cancer Society, colorectal cancer is expected to be the 4th most diagnosed cancer in Canada in 2024. As per estimates provided by the Canadian Cancer Society, 25,200 Canadians were diagnosed with colorectal cancer, which represented 10% of all new cancer cases in 2024.

Capsule endoscopy offers various benefits, such as painless visualization of gastrointestinal tract images, precise diagnosis, and faster results, contributing to their increased adoption in recent years. The launch of technologically advanced and innovative wireless capsules with enhanced features, including longer battery life, improved data storage capability, WiFi connectivity, ergonomic designs, easy transmission, higher frame rate, 360-degree panoramic view, and improved image quality, are driving the market growth during the forecast period. For instance, in November 2021, Medtronic received a U.S. FDA clearance for its PillCam Small Bowel 3 system for remote endoscopy procedures. The PillCam SB3 @HOME initiative incorporates Medtronic's PillCam technology with Amazon's logistics, aiming to deliver timely and precise patient outcomes right from the comfort of their homes
As awareness of the clinical benefits of capsule endoscopy, patient convenience, and diagnostic accuracy continues to rise, more hospitals and healthcare providers are incorporating capsule endoscopy into their gastroenterology services. This adoption is driven by the supportive reimbursement policies, and demand for minimally invasive diagnostic procedures, especially for conditions that are difficult to diagnose with traditional endoscopy. Hospitals are increasingly investing in capsule endoscopy technology as it provides detailed imaging of the entire small intestine. For instance, Medicare doesn't have a National Coverage Determination (NCD) for wireless capsule endoscopy (WCE). However, Local Coverage Articles (LCAs) and Local Coverage Determinations (LCDs) adhere to these applicable WCE procedures.
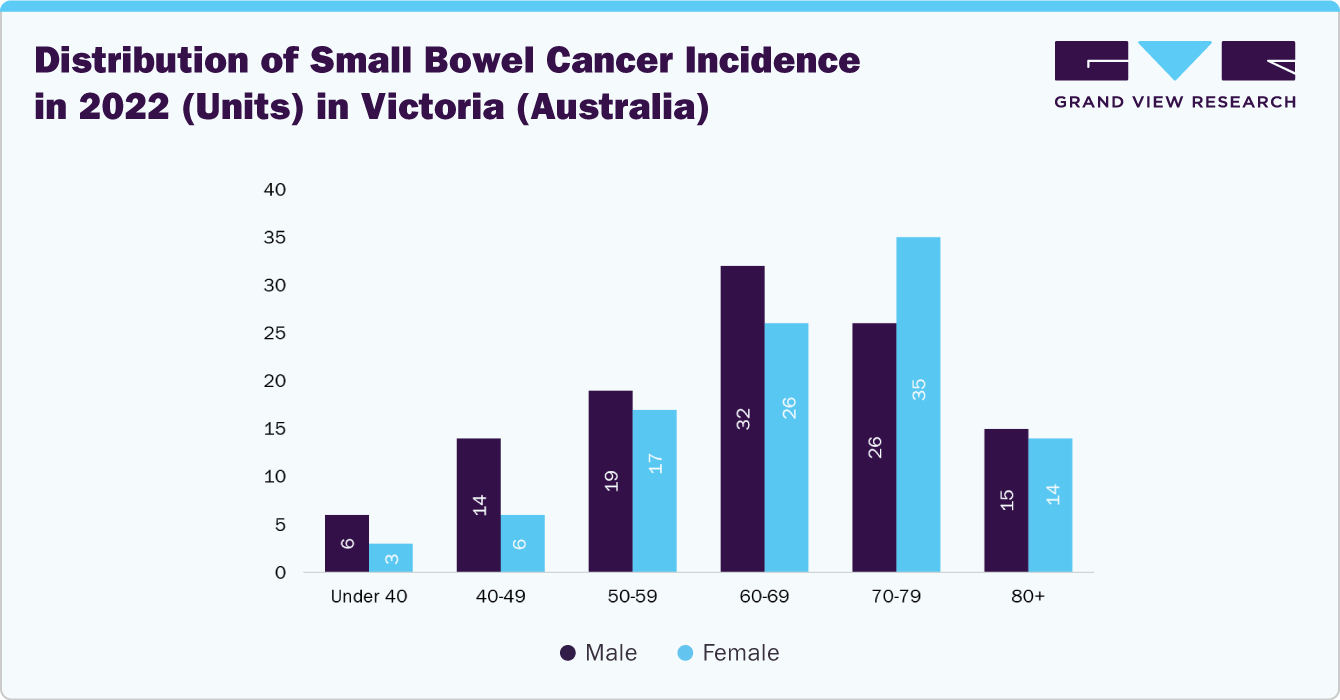
Capsule endoscopy is a non-invasive diagnostic technology that uses a swallowable, wireless capsule equipped with a miniature camera to capture high-resolution gastrointestinal (GI) tract images. It is widely adopted for detecting obscure gastrointestinal bleeding (OGIB), Crohn’s disease, small bowel tumors, colorectum cancers, esophagus cancer, and celiac disease, offering a patient-friendly alternative to conventional endoscopy. Moreover, according to Cancer Council Victoria (Australia), in 2022, a total of 213 people in Victoria were diagnosed with small bowel cancer. This included 101 females and 112 males, accounting for 47.4% and 52.6% of all small bowel cancer cases in the region.
Developing training programs and integrating capsule endoscopy into medical, educational, and gastroenterology fellowships fuel the adoption of capsule endoscopy. With the continuous development of advanced capsule technologies, including magnetically controlled capsules and AI-powered automated lesion detection, the need for structured training and certification programs is expected to rise further. This trend ensures improved diagnostic efficiency and strengthens the confidence of healthcare professionals in utilizing capsule endoscopy for routine and complex GI investigations, which drives market growth. For example, UEG (United European Gastroenterology) provides “A Primer In Capsule Endoscopy,” an online course designed to comprehensively introduce capsule endoscopy, the associated reporting software, and the possible complications encountered.
Market Concentration & Characteristics
The global capsule endoscopy industry t is characterized by a high degree of innovation, owing to the development of technologically advanced products and innovations that have enhanced diagnostic accuracy, improved patient comfort, and minimized risks associated with traditional endoscopes. For instance, in December 2024, the Asian Institute of Gastroenterology (AIG) Hospital introduced PillBotT, a remote-controlled (robotic) disposable endoscopy capsule developed by Endiatx, a U.S. medical innovator.
The market is characterized by medium-sized mergers and acquisition activity, owing to several factors, including the desire to expand the business to cater to the growing demand for capsule endoscopes to maintain a competitive edge.
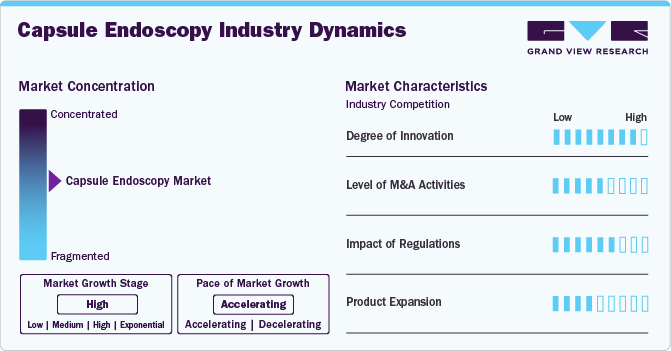
Capsule endoscopes must meet strict regulatory requirements to ensure high quality, safety, and effectiveness standards positively impact market growth. The U.S. Food and Drug Administration (FDA) regulates capsule endoscopy as a Class II device. Basic regulatory requirements for the manufacturing and distribution of medical devices in the U.S. are establishing registration, listing of medical devices, Premarket Approval (PMA) process, Investigational Device Exemption (IDE) for clinical research, regulating Quality System (QS), labeling requirements, and Medical Device Reporting.
Several market players are expanding their business by launching new products and getting approvals from regulatory authorities to strengthen their market position and expand their product portfolio. For instance, in May 2024, Medtronic received U.S. FDA clearance for the next-generation PillCam Genius SB capsule endoscopy kit.
Type Insights
By type, the small bowel capsule endoscopy segment dominated the market with the largest revenue share of 54.6% in 2024. The increasing demand for advanced diagnostic tools for these diseases to provide efficient and patient-friendly alternatives is anticipated to drive the segment growth in the market, which is expected to lead to the growing launch of new products in the market. For instance, in December 2024, Medtronic conducted its first patient procedure with the University of Miami Health System (UHealth)HeH by utilizing its next-generation PillCam Genius SB capsule endoscopy kit.
The colon capsule endoscopy segment is anticipated to grow at the fastest CAGR over the forecast period, owing to the increasing awareness regarding colon cancer and the emphasis on increasing colon screening in several countries across the world. Furthermore, the rising disposable income, rapid technological advancements in capsule endoscopy, growing demand for minimally invasive surgeries, and implementation of pivotal screening programs for effective cancer diagnosis are key factors promoting market growth. For instance, according to the government of Mexico, colorectal cancer ranks third and fourth in frequency among the Mexican population. Annually, approximately 8,700 new cases are diagnosed in the country.
Application Insights
By application, the OGIB (obscure GI tract bleeding) segment dominated the market with the largest revenue share of 45.4% in 2024. The technological advancements and development of artificial intelligence algorithms for image analysis, with companies working on AI-assisted platforms to streamline the interpretation of capsule endoscopy results, are anticipated to drive market growth. For instance, in January 2024, the U.S. FDA approved AnX Robotica’s AI-powered endoscopy tool to enhance the analysis of small bowel capsule endoscopy (SB-CE) images. This technology aims to assist clinicians in detecting suspected small bowel bleeding in adult patients, improving diagnostic accuracy and efficiency.
The small intestine tumor segment is anticipated to grow at the fastest CAGR over the forecast period. The increasing significance of capsule endoscopy in the safe and noninvasive diagnosis of small intestine tumors and related disorders is driving the growth of this segment. Moreover, clinical initiatives have emphasized capsule endoscopy's role in managing small intestine tumors. For instance, according to the American Cancer Society estimates, in 2024, around 13,920 new small intestine cancer cases were diagnosed in the U.S.
End-use Insights
By end-use, the hospitals segment dominated the market with a revenue share of 55.3% in 2024 and is expected to grow at the fastest rate during the forecast period. Hospitals have been at the forefront of implementing innovative capsule endoscopy technologies. For instance, in September 2024, AIIMS Nagpur inaugurated a new endoscopy room under its Department of Medical Gastroenterology. This expansion aims to provide advanced diagnostic and therapeutic services to patients in the region. Such developments are likely to drive the segment growth over the forecast period.
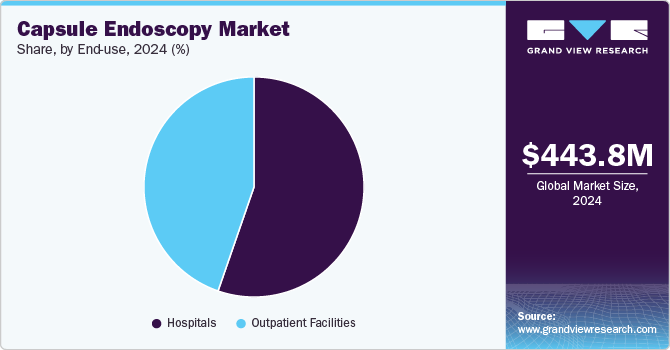
The outpatient facilities segment is expected to grow at a significant rate during the forecast period. Factors include the increasing preference for daycare and ambulatory surgery centers in colon screenings, which fuels the market growth. In addition, faster recovery time and minimal discomfort due to noninvasive endoscopic procedures accelerate the adoption of capsule endoscopy in outpatient facilities, which is anticipated to propel segment growth over the forecast period.
Regional Insights
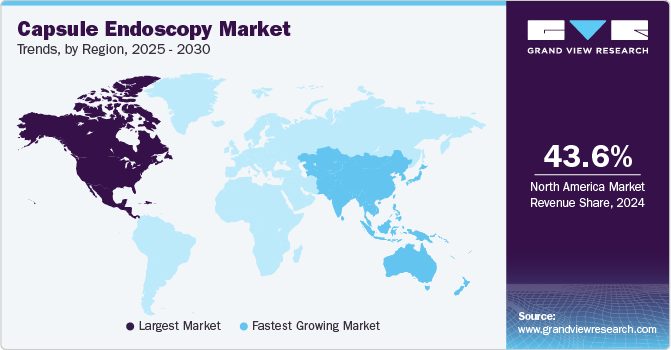
North America dominated the capsule endoscopy market with a revenue share of 43.6% in 2024. The high prevalence of several diseases, such as Gastrointestinal (GI) disorders, which include Crohn's disease, gastrointestinal bleeding, celiac disease, ulcerative colitis, colon polyps, or colon cancer, is increasing the need for endoscopic procedures for diagnosis and treatment in North America. For instance, according to data published by the Government of Canada, approximately 260,000 individuals in Canada suffer from inflammatory bowel disease.
U.S. Capsule Endoscopy Market Trends
The U.S. dominated the capsule endoscopy market in North America region in 2024. The growing preference for minimally invasive procedures across various medical specialties in the U.S., including gastroenterology, pulmonology, urology, gynecology, and otolaryngology, is driving the demand for endoscopes. Furthermore, many industry players in the U.S. initially pursued approval from the FDA to introduce their products in the country, promoting market growth. For instance, in January 2022, AnX Robotics received a U.S. FDA clearance for its expanded indications of NaviCam Small Bowel Video Capsule Endoscopy (SB).
Europe Capsule Endoscopy Market Trends
The capsule endoscopy market in Europe is expected to grow significantly over the forecast period. Factors such as the relatively less stringent medical device approval process in the region are primarily boosting the market. In addition, high disposable income, availability of well-established healthcare infrastructure, presence of developed economies, and availability of skilled professionals are some of the major factors propelling the market growth.
The capsule endoscopy industry in the Germany is expected to grow significantly during the forecast period. The increasing incidence of cancer in Germany is further expected to drive the demand for advanced diagnostic solutions such as screenings in the country. According to the Robert Koch Institute (Federal Ministry of Health), around one in eight incidence cancers in Germany affects the colon (large intestine) or rectum. In 2022, approximately 29,960 men and 24,650 women were newly diagnosed.
Asia Pacific Capsule Endoscopy Market Trends
The Asia Pacific capsule endoscopy industry is expected to register the fastest growth rate over the forecast period. The presence of a large number of patient pools with a range of cancer incidences, advanced healthcare systems with a strong focus on innovation and technological advancements, and growing investments by market players to expand their presence in the region are anticipated to propel market growth.
China capsule endoscopy market is anticipated to register considerable growth during the forecast period. Improved healthcare facilities and growing healthcare expenditures, various initiatives undertaken by private players are expected to drive market growth. For instance, Olympus China Medical Training & Education Center (C-TEC) is Olympus China's medical training base, established in three cities such as Shanghai, Beijing, and Guangzhou.
Latin America Capsule Endoscopy Market Trends
The Latin America capsule endoscopy industry is anticipated to witness considerable growth over the forecast period. The growing preference for minimally invasive surgeries over open surgeries and increasing awareness about using capsule endoscopy for various diagnostic and therapeutic procedures are expected to drive the market. Moreover, the presence of rapidly developing economies, such as Brazil and Argentina, primarily drives the market growth in Latin America.
Brazil capsule endoscopy industry is anticipated to register considerable growth during the forecast period. Favorable government initiatives and high demand for healthcare services drive the country's healthcare sector. Moreover, increasing companies' initiatives to tap opportunities in the Brazilian market, rising incidences of GI conditions, and strengthening distribution networks are a few of the key factors driving the market growth.
Middle East & Africa Capsule Endoscopy Market Trends
The Middle East and Africa capsule endoscopy market is anticipated to witness considerable growth over the forecast period. The MEA capsule endoscopy devices market is gaining immense traction due to the increased number of hospitals and the rising demand for minimally invasive procedures. Moreover, the World Gastroenterology Organisation (WGO) introduced opening endoscopy training centers in Lagos (Nigeria), Nairobi (Kenya), Blantyre (Malawi), and Addis Ababa (Ethiopia).
South Africa capsule endoscopy industry is anticipated to register considerable growth during the forecast period. Increasing healthcare spending and rising demand for advanced healthcare treatments are some of the factors further anticipated to boost the South Africa capsule endoscopy market during the forecast period. For instance, according to Standard Bank data, South Africa spends around 8% to 9% of its GDP on healthcare.
Key Capsule Endoscopy Company Insights
Key participants in the capsule endoscopy industry are focusing on devising innovative business growth strategies, such as expanding their product portfolios, partnerships and collaborations, mergers and acquisitions, and expanding their business footprints.
Key Capsule Endoscopy Companies:
The following are the leading companies in the capsule endoscopy market. These companies collectively hold the largest market share and dictate industry trends.
- CapsoVision
- Shangxian Minimal Invasive Inc.
- INTROMEDIC
- Medtronic
- Olympus
- AnX Robotics
- JINSHAN Science & Technology (Group) Co., Ltd.
- Check-Cap
- RF Co., Ltd.
- BioCam
Recent Developments
-
In January 2025, CapsoVision received a U.S. FDA clearance for its CapsoCam Plus, which is now approved for use in pediatric patients aged two and older. This significant milestone offers a comfortable and noninvasive diagnostic alternative for children that reduces the stress associated with traditional capsule endoscopy procedures.
-
In January 2024, AnX Robotics received a U.S. FDA clearance for its expanded indications of NaviCam Small Bowel Video Capsule Endoscopy (SB) in children aged 2 years and above and adults.
Capsule Endoscopy Market Report Scope
|
Report Attribute |
Details |
|
Market size value in 2025 |
USD 475.7 million |
|
Revenue forecast in 2030 |
USD 695.4 million |
|
Growth rate |
CAGR of 7.9% from 2025 to 2030 |
|
Actual data |
2018 - 2024 |
|
Forecast data |
2025 - 2030 |
|
Quantitative units |
Revenue in USD million/billion and CAGR from 2025 to 2030 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Type, application, end-use, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
|
Country scope |
U.S.; Canada; Mexico; U.K.; Germany; Spain; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait |
|
Key companies profiled |
CapsoVision; Shangxian Minimal Invasive Inc.; INTROMEDIC; Medtronic; Olympus; AnX Robotics; JINSHAN Science & Technology (Group) Co., Ltd.; Check-Cap; RF Co., Ltd.; BioCam |
|
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. Explore purchase options |
Global Capsule Endoscopy Market Report Segmentation
This report forecasts revenue growth and provides at global, regional, and country levels an analysis of the latest trends in each of the sub-segments from 2018 to 2030. For this report, Grand View Research has segmented the global capsule endoscopy market report based on type, application, end-use, and region:
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Small Bowel Capsule Endoscopy
-
Esophageal Capsule Endoscopy
-
Colon Capsule Endoscopy
-
Others
-
-
Application Outlook (Revenue, USD Million, 2018 - 2030)
-
OGIB (obscure GI tract bleeding)
-
Crohn’s disease
-
Small Intestine tumor
-
Others (Celiac,NSAID reactions)
-
-
End-use Outlook (Revenue, USD Million, 2018 - 2030)
-
Hospitals
-
Outpatient Facilities
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global capsule endoscopy market size was estimated at USD 443.8 million in 2024 and is expected to reach USD 475.7 million in 2025.
b. The global capsule endoscopy market is expected to grow at a compound annual growth rate of 7.9% from 2025 to 2030 to reach USD 695.4 million by 2030.
b. North America dominated the capsule endoscopy market with a share of 43.6% in 2024. This is attributable to a rising prevalence rate of GI disorders & colorectal cancer coupled with advanced healthcare infrastructure, the presence of favorable reimbursement policies, and the increasing adoption of minimally invasive capsule endoscopy by patients.
b. Some key players operating in the capsule endoscopy market include Given Imaging (Medtronic), IntroMedic Co., Ltd., Olympus Corporation, Chongqing Jinshan Science & Technology (Group) Co., Ltd, RF System lab, and CapsoVision.
b. Key factors that are driving the capsule endoscopy market growth include increasing prevalence of gastrointestinal diseases, colorectal cancer, demand for faster and accurate diagnostics tools, and the presence of supportive government initiatives coupled with rising global geriatric population base.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."




