- Home
- »
- Pharmaceuticals
- »
-
Breakthrough Therapy Designation Market Report, 2030GVR Report cover
![Breakthrough Therapy Designation Market Size, Share & Trends Report]()
Breakthrough Therapy Designation Market (2024 - 2030) Size, Share & Trends Analysis Report By Application (Oncology, Infectious Disease, Rare Disease, Autoimmune Disease), By End Use, By Region, And Segment Forecasts
- Report ID: GVR-2-68038-070-5
- Number of Report Pages: 90
- Format: PDF
- Historical Range: 2018 - 2022
- Forecast Period: 2024 - 2030
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Market Size & Trends
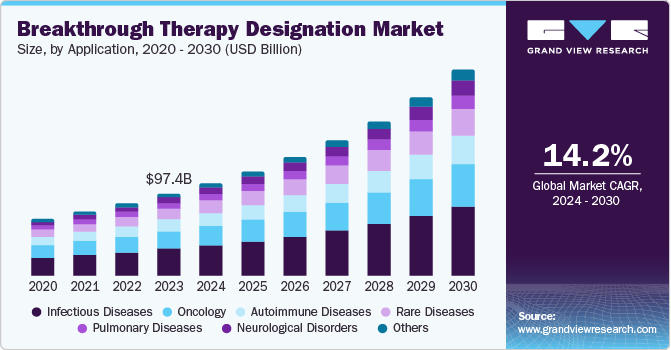
The global breakthrough therapy designation market size was valued at USD 97.4 billion in 2023 and is projected to grow at a CAGR of 14.2% from 2024 to 2030. It is a process designed to escalate the development and assessment of sanctioning of drugs & biologics that are proposed for treating severe diseases, whereas primary clinical evidence notifies that the drug determines considerable enhancement over existing therapy on a clinically significant endpoint. Furthermore, the BT (Breakthrough) designation lets pharma companies hasten the developmental process by offering additional support and assistance from the FDA and making medications available to the public faster.
Apart from breakthrough designation therapy, there are some important tools, all of which have been in place for many years, such as fast-track designation, accelerated approval, and priority review. All of these are inclined toward approving drugs used to treat serious disorders. Although these processes can reduce a drug's time to market, standard clinical testing is required for the development process, which usually involves three phases of large-scale and controlled trials.
The increasing prevalence of life-threatening conditions and the necessity for the rapid development of pipeline drugs are also some factors that propel the breakthrough therapy drug market. This is primarily driven by the significant unmet need for effective treatments for severe conditions currently available. Manufacturing companies are particularly attentive to drugs designated as breakthrough therapies due to the accelerated market access and higher returns on investment. Breakthrough therapy drugs often undergo less extensive clinical trials, which is a direct consequence of their market designation. The FDA's enhanced support for small-scale industries in research and development, including increased funding and expedited drug approval processes, is further stimulating the market for breakthrough therapy drugs. Collectively, these elements are pushing the breakthrough therapy drug market forward.
Further, innovative gene and cell therapies are offering new treatment decisions for previously untreatable illnesses, mainly drifting to more breakthrough therapy designations. Moreover, the regulatory support agencies are streamlining processes and offering assistance for breakthrough therapies to speed up their development and sanction. Another aspect leading the breakthrough therapy designations is the cross-sector collaborations between academic institutions, pharmaceutical companies, and research institutes that are lifting the upgradation and the advancement of new breakthrough therapies completely.
Application Insights
Infectious diseases dominated the application segment with a share of 33.5% in 2023 owing to an increase in the sales of popular drugs such as Harvoni and Sovaldi; there has also been a significant rise in the number of anti-infective medications receiving BT designation. For instance, PolyPid was granted breakthrough therapy designation by the FDA for D-PLEX100, which is aimed at preventing Surgical Site Infections in Colorectal Surgery.
The neurological disorders are expected to grow at a CAGR of 15.3% over the forecast period. It is becoming increasingly recognized that neurological disorders exhibit a wide range of variations and require individualized treatments that consider the patient's genetic, molecular, and clinical characteristics. A report by the World Health Organization (WHO) revealed that more than 1 billion people worldwide are impacted by neurological disorders, which are major contributors to mortality and disability. Common neurological disorders include Alzheimer's disease, Parkinson's disease, stroke, epilepsy, multiple sclerosis, migraine, and traumatic brain injury. Furthermore, the FDA has awarded breakthrough designations to various devices and diagnostics for neurological disorders, such as an autism screening tool and therapy, as well as the detection of Parkinson's disease.
End Use Insights
Hospitals led the market and accounted for the largest revenue share of 32.0% in 2023, owing to the large patient volume at the hospitals, which creates a significant demand for advanced treatments. In addition, hospitals have good relations with pharmaceutical companies, making it easy to access breakthrough therapies. The specialized departments of oncology or cardiology in hospitals have the developed and enhanced infrastructure with a number of resources to access at a time, which allows them to undertake critical treatments or trials.
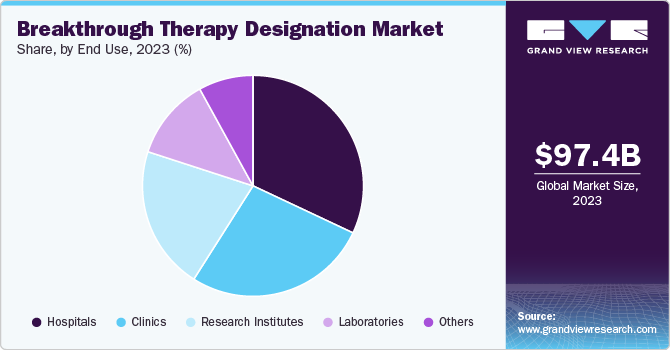
Clinics witnessed significant growth and are expected to grow at a CAGR of 14.5% over the forecast period. The main focus on improving and innovating specialized and personalized treatment options and increasing outpatient services as well as ambulatory care centers contributes to market growth. In addition, clinics are more agile and variable than hospitals, allowing them to adopt the latest treatment methods and technology. Furthermore, clinics leverage innovative health technologies and data analysis to enhance the care of patients and also to create new opportunities in breakthrough therapies.
Regional Insights
The North America breakthrough therapy designation market had the largest market share of 41.8% in 2023 attributed to intellectual property laws that incentivize innovations with data protection and copyright security. Furthermore, the availability of advanced healthcare services and an increase in chronic illnesses are the main reasons for dominating the market.
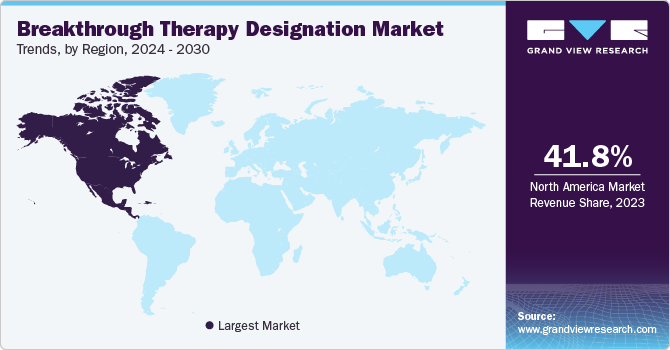
U. S. Breakthrough Therapy Designation Market Trends
The Breakthrough Therapy Designation Market in the U.S. dominated North America with the largest revenue share in 2023 driven by personalized medicine tailored to individual patient requirements, leading to specified therapies with higher efficiency and fewer side effects. Innovations in AI (Artificial Intelligence) are also being used to analyze large data sets to identify prospective breakthrough therapies rapidly and more accurately in accordance with the latest market trends.
Europe Breakthrough Therapy Designation Market Trends
Europe breakthrough therapy designation market is expected to witness significant growth due to improved infrastructure and reimbursement policies. The increasing adoption of innovative drugs by consumers is projected to drive the growth of the breakthrough therapy (BT) designation market in the region throughout the forecast period.
The breakthrough therapy designation market in Germany dominated Europe in 2023, driven by its well-established healthcare sector, robust developments in the research sector, and favorable regulatory environment. The strong biotechnology sector, augmented with top pharmaceutical companies, created a place for innovation and development in terms of breakthrough therapy designation. Germany’s regulatory authority, the Federal Institute for Drugs and Medical Devices had adopted a supportive framework for expedited review and approval of breakthrough therapies, fueling the market growth.
Spain breakthrough therapy designation market is expected to grow over the forecast period. The growth in individuals' per capita income, their inclination to invest in improved medical services, and the growing favor for innovative medications will contribute to the emergence of new growth prospects in this market in the future.
Asia Pacific Breakthrough Therapy Designation Market Trends
The breakthrough therapy designation market in Asia Pacific is expected to grow significantly, with a CAGR of 15.5% over the forecast period owing to ongoing research on the diseases and rising R&D investments in this region.
China breakthrough therapy designation market is expected to grow rapidly growth, due to urgent requirements to face the rapid growth of chronic illnesses, such as cancer, diabetes, or cardiac diseases. In addition, the government has taken initiatives to reform the healthcare system and drive innovation in the pharmaceutical sector, which has created a suitable environment for the growth of the breakthrough therapy designation market. Furthermore, increasing developments in the biotechnology segment of China, aligned with the rising demand for personalized and targeted therapies, drive the market.
The growth of the breakthrough therapy designation market in India is driven as a result of a large volume of dealing with chronic diseases, combined with the government’s assistance to enhance healthcare and to make access available to advanced treatment options in the current generation. Furthermore, the rising prevalence of rare diseases and disorders, backed by a lack of efficient treatments, led to a high demand for breakthrough therapies.
Key Breakthrough Therapy Designation Company Insights
Some of the key companies in the breakthrough therapy designation market include F. Hoffmann-La Roche Ltd; Novartis AG; Pfizer, Inc.; AbbVie, Inc.; Bristol-Myers Squibb Company; Gilead Sciences, Inc.; Sanofi; Regeneron Pharmaceuticals Inc.; AstraZeneca; Boehringer Ingelheim GmbH in the market are focusing on development & to gain a competitive edge in the industry.
-
Novartis AG is a healthcare company that focuses on the innovation, development, manufacture, and marketing of prescription and generic pharmaceutical and eye care products. It offers drugs for treating cancer, cardiovascular diseases, dermatological conditions, neurological disorders, ophthalmic and respiratory diseases, and others.
-
Sanofi is a healthcare company that deals with the discovery, development, manufacturing, and marketing of a variety of medicines and vaccines. It also offers consumer healthcare products for digestion, allergy, cough, cold, flu, and sinus, pain, women’s health, and vitamins, minerals, and supplements.
Key Breakthrough Therapy Designation Companies:
The following are the leading companies in the breakthrough therapy designation market. These companies collectively hold the largest market share and dictate industry trends.
- F. Hoffmann-La Roche Ltd
- Novartis AG
- Pfizer, Inc.
- AbbVie, Inc.
- Bristol-Myers Squibb Company
- Gilead Sciences, Inc.
- Sanofi
- Regeneron Pharmaceuticals Inc.
- AstraZeneca
- Boehringer Ingelheim GmbH
Recent Developments
- In May 2024, Novartis announced that the FDA had granted Scemblix (asciminib) Breakthrough Therapy designation for treating adult patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase (Ph+ CML-CP).
Breakthrough Therapy Designation Market Report Scope
Report Attribute
Details
Market size value in 2024
USD 109.5 billion
Revenue forecast in 2030
USD 242.7 billion
Growth rate
CAGR of 14.2% from 2024 to 2030
Base year for estimation
2023
Historical data
2018 - 2022
Forecast period
2024 - 2030
Quantitative units
Revenue in USD billion and CAGR from 2024 to 2030
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Application, end use, region
Regional scope
North America, Europe, Asia Pacific, Latin America, MEA
Country scope
U.S., Canada, Mexico, UK, Germany, France, Italy, Spain, Denmark, Sweden, Norway, Japan, China, India, Australia, South Korea, Thailand, Brazil, Argentina, South Africa, Saudi Arabia, UAE, Kuwait
Key companies profiled
F. Hoffmann-La Roche Ltd; Novartis AG; Pfizer, Inc.; AbbVie, Inc.; Bristol-Myers Squibb Company; Gilead Sciences, Inc.; Sanofi; Regeneron Pharmaceuticals Inc.; AstraZeneca; Boehringer Ingelheim GmbH
Customization scope
Free report customization (equivalent to up to 8 analysts' working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Breakthrough Therapy Designation Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the breakthrough therapy designation market report based on application, end use, and region:
-
Application Outlook (Revenue, USD Billion, 2018 - 2030)
-
Oncology
-
Infectious Diseases
-
Rare Diseases
-
Autoimmune Diseases
-
Pulmonary Diseases
-
Neurological Disorders
-
Others
-
-
End Use Outlook (Revenue, USD Billion, 2018 - 2030)
-
Hospitals
-
Clinics
-
Research Institutes
-
Laboratories
-
Others
-
-
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa (MEA)
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










