
AI In Medical Writing Market Size, Share, & Trends Analysis Report By Type (Clinical Writing, Type Writing), By End-use (Medical Devices, Pharmaceutical), By Region, And Segment Forecasts, 2025 - 2030
- Report ID: GVR-4-68040-097-9
- Number of Report Pages: 100
- Format: PDF
- Historical Range: 2018 - 2024
- Forecast Period: 2025 - 2030
- Industry: Healthcare
AI In Medical Writing Market Size & Trends
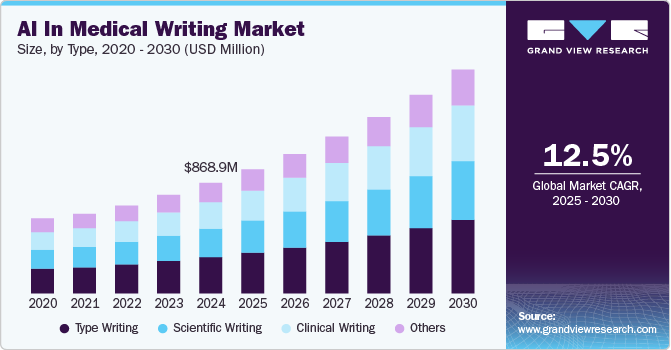
The global AI in medical writing market size was estimated at USD 868.99 million in 2024 and is projected to grow at a CAGR of 12.52% from 2025 to 2030. The adoption of AI in medical writing is significantly accelerating the production of regulatory documents, clinical study reports, and scientific publications. AI-driven tools streamline repetitive tasks, such as data extraction, summarization, and template generation, reducing human error and enabling faster project turnarounds.
For instance, in June 2024, TrialAssure, a company focused on clinical trial disclosure, data sharing, and transparency, unveiled TrialAssure LINK AI 2.0. The updated version is tailored to support pharmaceutical and biotechnology companies, clinical research organizations, and service providers in efficiently drafting common regulatory and medical writing documents using advanced AI technology.
Artificial intelligence (AI) ensures compliance with stringent regulatory requirements by offering accurate, consistent, and error-free documentation. Natural language processing (NLP) algorithms are equipped to detect inconsistencies and align content with guidelines set by agencies like the FDA and EMA. This capability is critical in the medical writing industry, where non-compliance can lead to delays or rejections. The assurance of producing high-quality, regulation-compliant content has driven organizations to adopt AI technologies to meet evolving standards.
Integrating AI with data analytics has allowed real-time insights, enabling dynamic content creation tailored to specific clinical findings or market trends. These tools can interpret large volumes of clinical trial data and translate them into structured and coherent documents. AI-driven platforms enhance the ability of medical writers to produce highly targeted and scientifically robust content, which is essential for gaining a competitive edge. This integration between AI and analytics fosters market growth across the pharmaceutical and biotech sectors.
AI enables the creation of personalized medical content, addressing the specific needs of healthcare professionals, patients, and regulatory bodies. Customization is increasingly valued in the industry as it enhances the communication of complex medical information. For instance, AI algorithms can generate different versions of a single document to cater to diverse audiences. This flexibility supports precision in communication, making AI an indispensable tool for companies looking to engage stakeholders effectively.
The adoption of AI in medical writing is rapidly growing in emerging markets due to increased investments in pharmaceutical and clinical research. Countries in Asia-Pacific, the Middle East, and Latin America are leveraging AI to overcome challenges such as skill shortages and high documentation demand. Governments and private organizations are funding AI-driven solutions to boost their healthcare and research capabilities. The expansion of AI applications in these regions highlights its pivotal role in advancing global medical writing practices, further propelling the market’s growth.
Case Study Moderna develops 750 GPTs, significantly accelerating AI adoption
OpenAI recently published a case study highlighting their collaboration with Moderna, showcasing how the pharmaceutical giant is leveraging ChatGPT Enterprise to transform operations. By integrating AI across departments, Moderna is reimagining processes in legal, research, manufacturing, and commercial functions, aiming for an organization-wide redesign powered by AI.
Moderna’s leadership is committed to this transformation, with their CEO stating:
"We believe very profoundly at Moderna that ChatGPT and what OpenAI is doing is going to change the world. We’re looking at every business process—and thinking about how to redesign them with AI."
To achieve this vision, Moderna rolled out ChatGPT Enterprise to thousands of employees, setting an ambitious goal of 100% adoption and proficiency in generative AI within six months. Results indicate significant progress:
-
employees across the organization created 750 custom GPTs.
-
40% of weekly active users developed their GPTs tailored to their workflows.
-
On average, each user engaged in 120 weekly conversations with ChatGPT.
This integration highlights the potential of AI in medical writing, a critical function in the pharmaceutical industry. AI-enabled tools can assist in drafting clinical trial documentation, summarizing research data, automating compliance reports, and streamlining communication with regulatory bodies. The ability to create custom GPTs allows employees to design solutions for specific medical writing challenges, such as adapting complex scientific content into reader-friendly formats or ensuring consistency in style and tone across documents.
Market Concentration & Characteristics
The degree of innovation in the AI in medical writing industry is exceptionally high, driven by advancements in natural language processing (NLP) and machine learning. These technologies enable real-time drafting, editing, and summarization of complex medical documents. Custom AI solutions are increasingly tailored for regulatory compliance, clinical trial documentation, and scientific writing, enhancing efficiency, accuracy, and scalability across the pharmaceutical and biotech sectors.
The AI in medical writing industry is witnessing a moderate yet steadily increasing level of mergers and acquisitions (M&A) activity. Companies are pursuing strategic acquisitions to enhance AI capabilities, integrate innovative technologies, and expand their service offerings. For instance, in October 2023, Springer Nature strengthened its AI capabilities with the acquisition of Slimmer AI's Science division. As technology continues to enhance the publishing of trusted scientific content, the global academic publisher announced a definitive agreement to integrate the Netherlands-based Slimmer AI’s Science division into its operations. These M&A efforts aim to drive efficiency, streamline regulatory processes, and strengthen market positions in the rapidly evolving medical writing domain.

Regulations significantly impact AI in medical writing industry by ensuring compliance, safety, and ethical use of AI technologies. Strict regulatory frameworks necessitate transparency, data privacy, and adherence to industry standards, which can slow innovation but enhance trust and reliability. Regulatory approvals for AI tools, particularly in pharmaceutical and healthcare applications, shape market growth, driving developers to prioritize quality and accountability while fostering confidence among stakeholders in adopting these advanced solutions.
The growing need for efficient and accurate documentation in the pharmaceutical and healthcare industries drives product expansion in the AI in medical writing industry. Companies are introducing advanced AI-driven tools that streamline regulatory submissions, clinical trial reporting, and scientific content creation. Features like multilingual support, generative AI integration, and real-time collaboration enhance productivity.
Geographical expansion in the AI in medical writing industry is fueled by increasing global demand for streamlined medical documentation and regulatory compliance. Companies are targeting emerging markets in Asia-Pacific, Latin America, and the Middle East, where pharmaceutical and clinical research activities are rising. By establishing regional offices, forming strategic partnerships, and offering localized AI solutions, businesses are addressing diverse regulatory requirements and boosting adoption across global healthcare and life sciences sectors.
Type Insights
Based on type, the market is segmented into clinical writing, typewriting, scientific writing, and others. The typewriting segment led the market in 2024, accounting for the largest revenue share of 32.89%. This segment plays a crucial role throughout the entire product development process, particularly in scientific documentation. Typewriting is essential for the proper organization and presentation of data from clinical trials, which is required for regulatory submissions and product authorization. Upon receiving approval for a drug or product, post-approval regulatory certification becomes mandatory, necessitating additional paperwork and documentation. Due to the stringent regulations and extensive documentation requirements, there has been a surge in demand for efficient and accurate medical writing, further driving the growth of this segment.
The clinical writing segment, on the other hand, is expected to experience the fastest CAGR of 13.30% during the forecast period. Clinical writing is regularly used by medical professionals for various purposes, including patient documentation, clinical trial reporting, and regulatory submissions. Clinical writers need to have a comprehensive understanding of the clinical field, medical terminology, and the language used in medical documentation. In addition, they must be able to effectively communicate with both clinical professionals and regulatory bodies, which requires expertise in understanding the target audience and purpose of the writing.
End Use Insights
The end use segment comprises medical devices, pharmaceuticals, biotechnology, and others. In 2024, the pharmaceutical segment led the market, capturing a revenue share of 33.33%. Pharmaceutical companies make substantial investments in research and development (R&D) to create innovative drugs and treatments. These efforts generate extensive data from clinical trials, preclinical studies, and scientific literature, providing a rich source for training AI models used in medical writing tasks.
The adoption of AI-enabled solutions has significantly streamlined the medical writing process, with many companies reporting notable reductions in the time required for these tasks. Specifically, AI-powered tools can automate substantial portions of the Clinical Study Report (CSR) writing process, enhancing efficiency and accuracy. The time savings achieved through CSR automation solutions vary by provider. For instance, ZYLiQ.ai, a player in the industry, claims its solutions can reduce the time medical writers spend on tasks by 60-70%. This growing reliance on AI-driven technologies underscores the pharmaceutical sector’s pivotal role in advancing the adoption of AI in medical writing.
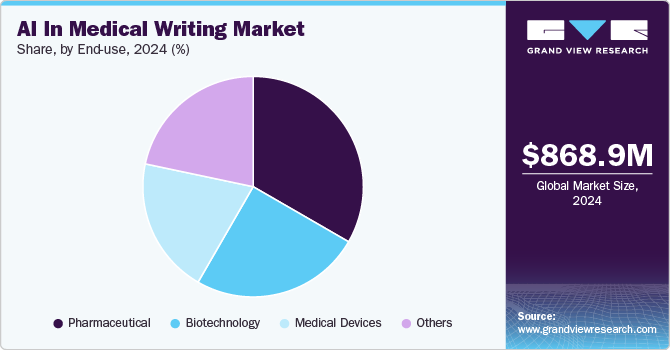
The medical devices segment is projected to experience the fastest CAGR of 13.59% during the forecast period. Medical writing is a critical component of the medical device development process, involving preparing documents such as clinical trial protocols, investigator brochures, patient information leaflets, and regulatory submissions. These documents provide essential insights into device safety, efficacy, and usage. Furthermore, medical writing documents the scientific rationale, regulatory compliance, and clinical data supporting device development. This ensures a clear narrative, aiding regulatory approvals and establishing credibility in the device's design, development, and effectiveness.
Regional Insights
North America AI in medical writing market is experiencing robust growth, driven by advancements in AI technology and its application in medical writing processes. According to a Science Direct report published in September 2024, AI can streamline manuscript preparation by aligning content with journal guidelines and expediting the peer-review process. This time-saving capability enhances efficiency, enabling researchers and writers to focus on critical tasks, fueling market expansion across the region.
U.S. AI In Medical Writing Market Trends
The U.S. AI in medical writing market is growing rapidly, driven by technological advancements and innovative solutions. Cutting-edge AI tools streamline regulatory writing, data analysis, and content generation, improving accuracy and efficiency. For instance, in August 2023, Celegence introduced CAPTIS Copilot, an AI-driven compliance solution for medical device and diagnostic manufacturers. The platform is designed to streamline regulatory processes, including medical writing tasks such as crafting clinical evaluation reports, regulatory submissions, and post-market surveillance documentation. Leveraging advanced AI capabilities, CAPTIS Copilot enhances document accuracy, compliance, and efficiency, ensuring manufacturers meet stringent global regulatory standards.
Europe AI In Medical Writing Market Trends
AI in medical writing market in Europe is expanding due to several key factors, including robust investment in AI research, strong pharmaceutical and biotech industries, and increasing adoption of digital transformation in healthcare. Advanced AI solutions enhance compliance with stringent European regulatory frameworks, streamline document creation, and improve efficiency. Collaborative efforts among industry players further drive innovation, solidifying Europe as a leader in integrating AI into medical writing processes.
The UK AI in medical writing market is growing due to the technology's ability to save time and simplify complex tasks. AI-powered tools streamline the creation of regulatory and scientific documents, reducing manual effort. Their user-friendly interfaces make them accessible to professionals across sectors, enhancing productivity and supporting the UK's thriving pharmaceutical and biotechnology industries.
AI in medical writing market in Germany is witnessing growth driven by innovation and the rising demand for scientific writing solutions. The introduction of AI tools like Springer Nature's Curie in October 2023 highlights this trend. Curie assists researchers in crafting scientific documents efficiently, addressing the increasing need for precision and speed in Germany's dynamic pharmaceutical and research sectors.
Asia Pacific AI In Medical Writing Market Trends
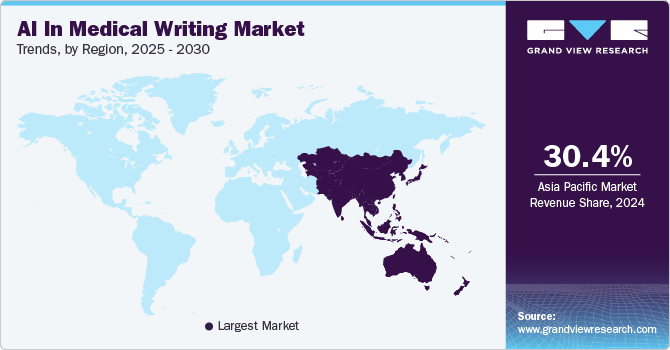
The AI in medical writing market in the Asia Pacific dominated with a revenue share of 30.41% and is growing rapidly, fueled by increasing demand for efficient and accurate scientific writing solutions. Rising pharmaceutical research activities and a growing need to streamline regulatory documentation processes are driving adoption. AI tools offer time-saving benefits and enhanced accuracy, making them indispensable in meeting the region's expanding medical and scientific communication requirements.
AI in medical writing market in Japan is experiencing significant growth, driven by advancements in AI technology and the nation's focus on precision and innovation. AI-powered tools streamline scientific documentation, improve accuracy, and reduce time spent on regulatory submissions, making them vital for Japan's expanding pharmaceutical and biotechnology industries.
AI in medical writing market in China is growing rapidly, driven by the increased adoption of AI tools to enhance productivity and streamline scientific writing processes. For instance, a 2023 Springer Nature report highlighted that 90% of Chinese researchers who used AI-assisted tools during trials advanced their manuscripts to peer review, resulting in a 14% rise in published articles.
Key AI In Medical Writing Company Insights
Some major players catering to the AI in medical writing industry are Parexel International (MA) Corporation; Trilogy Writing & Consulting GmbH; Freyr; Cactus Communications; GENINVO; IQVIA Inc.; ICON plc; Syneos Health; among others. There are various small and large manufacturers offering products, resulting in intense competition among the manufacturers. The industry players are increasing their focus on strategic partnerships such as mergers and acquisitions, new product launches & partnerships & collaborations to get maximum revenue share in this sector.
Key AI In Medical Writing Companies:
The following are the leading companies in the AI in medical writing market. These companies collectively hold the largest market share and dictate industry trends.
- Parexel International (MA) Corporation
- Certara, Inc.
- Trilogy Writing & Consulting GmbH
- Freyr
- Cactus Communications
- GENINVO
- IQVIA Inc.
- ICON plc
- Syneos Health
- IBM
- Teladoc Health, Inc.
- SmarterDx
- Abridge Al, Inc.
- Suki AI, Inc.
- Movano
- Heidi
- Corti
- Tortus AI.
- Nabla Technologies
Recent Developments
-
In June 2024, Certara, Inc. introduced its next-generation CoAuthor regulatory writing software, an innovative platform tailored for medical writers. CoAuthor integrates generative AI, pre-designed templates, Microsoft Word compatibility, and structured content authoring tools. This advanced solution streamlines the creation of regulatory documents, ensuring efficiency while upholding a "human-in-the-loop" approach to maintain quality and oversight in AI-assisted writing.
-
In July 2024, Cognizant announced a partnership with Yseop, a leading AI software company, to accelerate and scale scientific content delivery in the biopharma industry using generative AI. By leveraging Yseop Copilot to revolutionize medical writing in life sciences, the collaboration aims to boost productivity in drug development and expedite the launch of life-saving treatments. Cognizant is committed to driving Yseop's value and adoption across the life sciences sector, further advancing the digital transformation powered by AI.
-
In October 2023, Springer Nature introduced a new AI-powered in-house writing assistant designed to assist researchers, especially those whose first language is not English, in enhancing their scientific writing. Studies indicate that non-native English-speaking scientists require 51% more time to write a research paper, creating disparities in global research. This initiative aims to bridge the gap, fostering equal opportunities for high-quality research submissions worldwide.
AI In Medical Writing Market Report Scope
|
Report Attribute |
Details |
|
Market size value in 2025 |
USD 975.65 million |
|
Revenue forecast in 2030 |
USD 1,760.02 million |
|
Growth rate |
CAGR of 12.52% from 2025 to 2030 |
|
Actual data |
2018 - 2024 |
|
Forecast period |
2025 - 2030 |
|
Quantitative units |
Revenue in USD million and CAGR from 2025 to 2030 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Type, end use, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
|
Country scope |
U.S.; Canada; Mexico; UK; Germany; Spain; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait |
|
Key companies profiled |
Parexel International (MA) Corporation; Trilogy Writing & Consulting GmbH; Freyr; Cactus Communications; GENINVO; IQVIA Inc.; ICON plc; Syneos Health; IBM; Teladoc Health, Inc.; SmarterDx; Abridge AI, Inc.; Suki AI, Inc.; Movano; Heidi; Corti; Tortus AI; Nabla Technologies, Certara, Inc. |
|
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. Explore purchase options |
Global AI In Medical Writing Market Report Segmentation
This report forecasts revenue growth and provides at global, regional, and country levels an analysis of the latest trends in each of the sub-segments from 2018 to 2030. For this report, Grand View Research has segmented the global AI in medical writing market report based on type, end use, and region:
-
Type Outlook (Revenue, USD Million, 2018 - 2030)
-
Clinical Writing
-
Type Writing
-
Scientific Writing
-
Others
-
-
End Use Outlook (Revenue, USD Million, 2018 - 2030)
-
Medical Devices
-
Pharmaceutical
-
Biotechnology
-
Others
-
-
Regional Outlook (Revenue, USD Million, 2018 - 2030)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global AI in medical writing market accounted for USD 868.99 million in 2024 and is expected to reach USD 975.65 million in 2025.
b. The global AI in medical writing market is expected to grow at a CAGR of over 12.52% during the forecast period to reach USD 1,760.02 million in 2030.
b. The type writing segment dominated the market for AI in medical writing and accounted for the largest revenue share of 32.89% in 2024. This growth is driven by rising demand for efficiency in documentation, regulatory compliance, advancements in natural language processing, and increasing adoption by pharmaceutical and healthcare organizations.
b. Some of the key players operating in the AI in medical writing market include: • Parexel International (MA) Corporation • Certara, Inc. • Trilogy Writing & Consulting GmbH • Freyr • Cactus Communications • GENINVO • IQVIA Inc. • ICON plc • Syneos Health • IBM • Teladoc Health, Inc. • SmarterDx • Abridge Al, Inc. • Suki AI, Inc. • Movano • Heidi • Corti • Tortus AI. • Nabla Technologies
b. The AI in medical writing market is driven by increasing demand for precision in regulatory documentation, cost-efficient content generation, advancements in natural language processing, and growing adoption of AI in healthcare communication.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."




