- Home
- »
- Medical Devices
- »
-
Anastomosis Devices Market Size, Industry Report, 2033GVR Report cover
![Anastomosis Devices Market Size, Share & Trends Report]()
Anastomosis Devices Market (2026 - 2033) Size, Share & Trends Analysis Report By Product (Disposable, Reusable), By Application (Cardiovascular Surgery, Gastro-intestinal Surgery), By End Use, By Region, And Segment Forecasts
- Report ID: GVR-2-68038-138-2
- Number of Report Pages: 180
- Format: PDF
- Historical Range: 2021 - 2024
- Forecast Period: 2026 - 2033
- Industry: Healthcare
- Report Summary
- Table of Contents
- Segmentation
- Methodology
- Download FREE Sample
-
Download Sample Report
Anastomosis Devices Market Summary
The global anastomosis devices market size was estimated at USD 4.41 billion in 2025 and is projected to reach USD 7.07 billion by 2033, growing at a CAGR of 6.1% from 2026 to 2033. An estimated 310 million major surgeries are performed annually, with the U.S. accounting for 40 to 50 million of these operations.
Key Market Trends & Insights
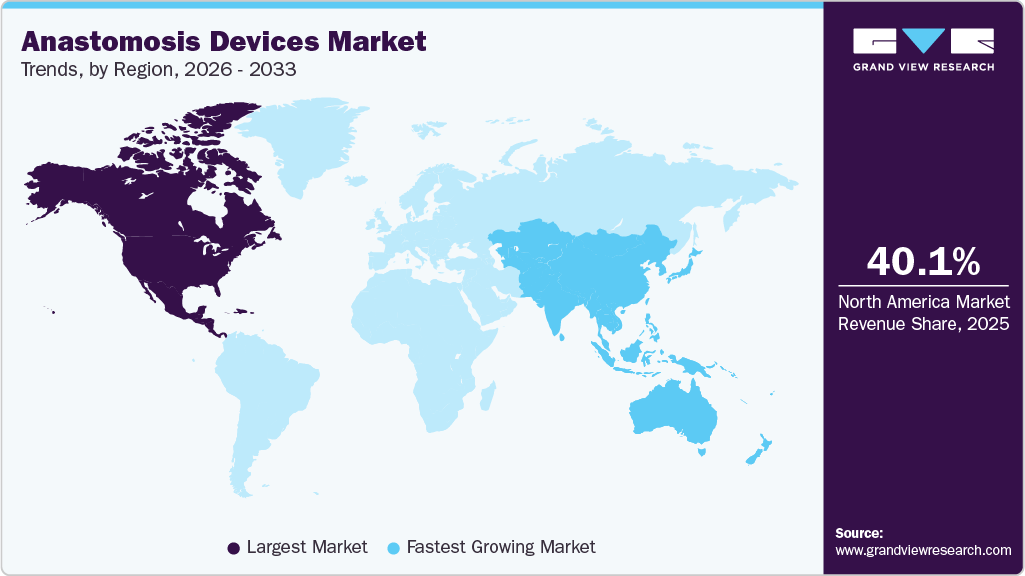
- North America dominated the global anastomosis device market with the largest revenue share of 40.1% in 2025.
- The anastomosis device industry in the U.S. accounted for the largest market revenue share in North America in 2025.
- By product, the disposable segment led the market with the largest market share of 88.5% in 2025.
- By application, the cardiovascular surgery segment accounted for the largest market revenue share in 2025.
- By end use, the hospitals segment accounted for the largest market revenue share in 2025.
Market Size & Forecast
- 2025 Market Size: USD 4.41 Billion
- 2033 Projected Market Size: USD 7.07 Billion
- CAGR (2026-2033): 6.1%
- North America: Largest market in 2025
- Asia Pacific: Fastest growing market
This escalation is largely propelled by an aging population and increasing cases of lifestyle-related diseases, particularly cardiovascular conditions and cancer. As surgical intervention becomes a standard necessity in addressing these health challenges, the demand for effective anastomosis devices has escalated, indicating a market poised for substantial expansion.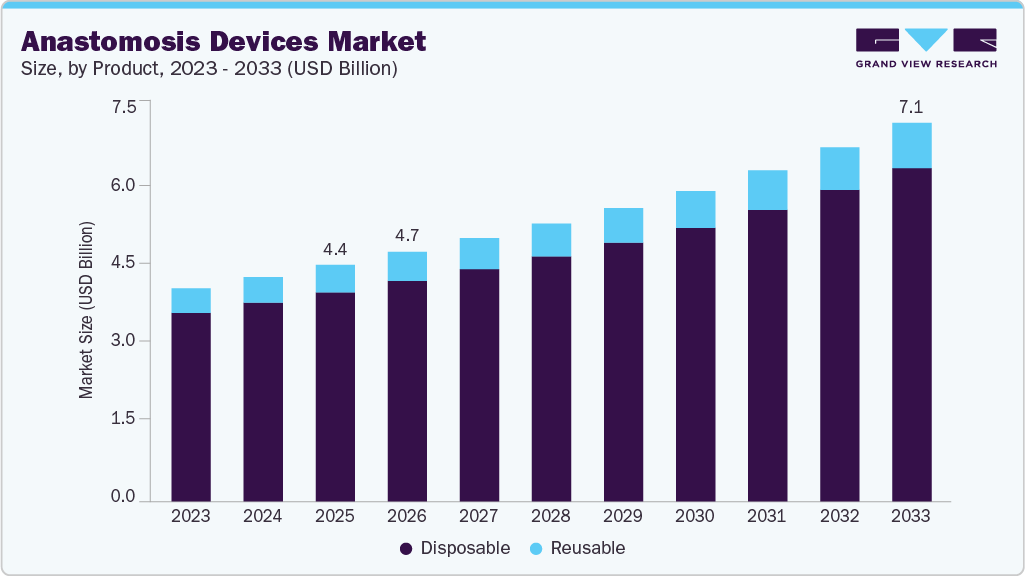
Supporting this trend is the rising prevalence of chronic diseases, which is becoming a significant health burden worldwide. Cardiovascular diseases, responsible for approximately 17.9 million annual fatalities, remain a leading cause of mortality. Concurrently, the oncology sector is grappling with approximately 20 million new cancer diagnoses per year. The correlation between an uptick in chronic ailments and the concomitant need for surgical procedures utilizing anastomosis devices suggests a deep-rooted market demand that is unlikely to abate in the coming years.
Technological advancements are also playing a pivotal role in the evolution of the anastomosis devices industry. Innovations in minimally invasive surgical techniques enhance patient outcomes and streamline operational efficiencies, leading to shorter recovery times and a reduction in postoperative complications. These enhancements make the adoption of anastomosis devices increasingly favorable for both healthcare providers and patients, thereby driving market demand and fostering a more competitive landscape within the medical devices sector.
The growing focus on patient outcomes and healthcare expenditure strengthens the market dynamics. North America, representing a significant share of the anastomosis devices industry, benefits from an advanced healthcare infrastructure backed by substantial financial investments. Forecasts indicate a continued upward trajectory in healthcare spending, further amplifying the market’s potential. As healthcare systems worldwide prioritize improving quality of life and recovery rates, the adoption of innovative anastomosis devices is expected to gain momentum, ensuring sustained growth within this critical segment of the medical device industry.
Product Insights
The disposable products segment led the market with the largest revenue share of 88.5% in 2025, due to the availability of a wide range of disposable anastomotic products and increased market penetration. The majority of key players are involved in the production of disposable types of products. These products reduce the risk of cross-contamination, Surgical Site Infections (SSI), and other Healthcare-associated Infections (HAI). Another advantage of disposable anastomotic devices over reusable ones is that they reduce reprocessing costs and speed up the process. Some of the commercially available disposable anastomosis devices include Valtrac, DST series EEA staplers, Mitroflow Valsalva Conduit, Endo GIA Reload with Tri-Staple Technology and Endo Stitch by Covidien, Ltd. Medtronic’s Multifire Endo TA 30 Staplers and Reloads are the disposable reloadable laparoscopic stapler for resection, transection, and anastomosis with titanium staples which is designed to provide precise and accurate staple formation, ensuring secure closure of tissues during surgical procedures.
The reusable products segment is expected to grow at the fastest CAGR of 6.7% over the forecast period, driven by the environmentally friendly and cost-effective benefits associated with reusable instruments. Reusable anastomosis devices are medical instruments that can be sterilized and used multiple times, which generates less waste. They are often used in surgical procedures that require a more robust and long-lasting connection between the two structures. The cost-benefit analysis, driven by the multiple uses of the same device, is expected to fuel the market during the forecast period.
Application Insights
The cardiovascular surgery segment led the market with the largest revenue share of 51.0% in 2025. Anastomosis devices are often used in cardiovascular surgeries to connect blood vessels during surgical procedures, as they reduce the risk of complications. The growth of the cardiovascular surgery segment is attributable to the high prevalence of cardiovascular disease, the rising geriatric population, and the advancements in surgical techniques and technology. Cardiovascular diseases such as coronary artery disease, peripheral artery disease, and vascular diseases are the leading causes of death worldwide.
The gastrointestinal surgery segment is expected to register at the fastest CAGR of 6.4% over the forecast period. The prevalence of gastrointestinal disorders, such as inflammatory bowel disease, colorectal cancer, and diverticulitis, has contributed to the increasing demand for gastrointestinal surgery. A study by the American Gastroenterological Association found that 40% of individuals worldwide have Functional Gastrointestinal Disorders (FGIDs). Anastomosis devices are used in gastrointestinal surgery to make it safer, more effective, and less invasive. They enable surgeons to perform complex procedures with greater precision and accuracy, leading to improved patient outcomes.
End Use Insights
The hospitals segment led the market with the largest revenue share of 57.5% in 2025, driven by the availability of advanced medical equipment, skilled professionals who can perform surgeries efficiently, and superior patient care. People prefer to undergo surgeries in hospitals because they perceive it as a safer environment and have access to emergency medical care if needed. Moreover, medical insurance coverage and reimbursements play a significant role in hospital surgeries, contributing to the largest revenue share in the market. According to the data from the Brazilian Federation of Hospitals (FBH) and the National Confederation of Health (CNSaúde), out of 7,191 hospitals in Brazil, 62% are privately owned. As of 2022, the country boasts 710 health insurance providers, 427,097 hospital beds, 546,000 physicians, 402,000 dentists, and 90,9000 drugstores.
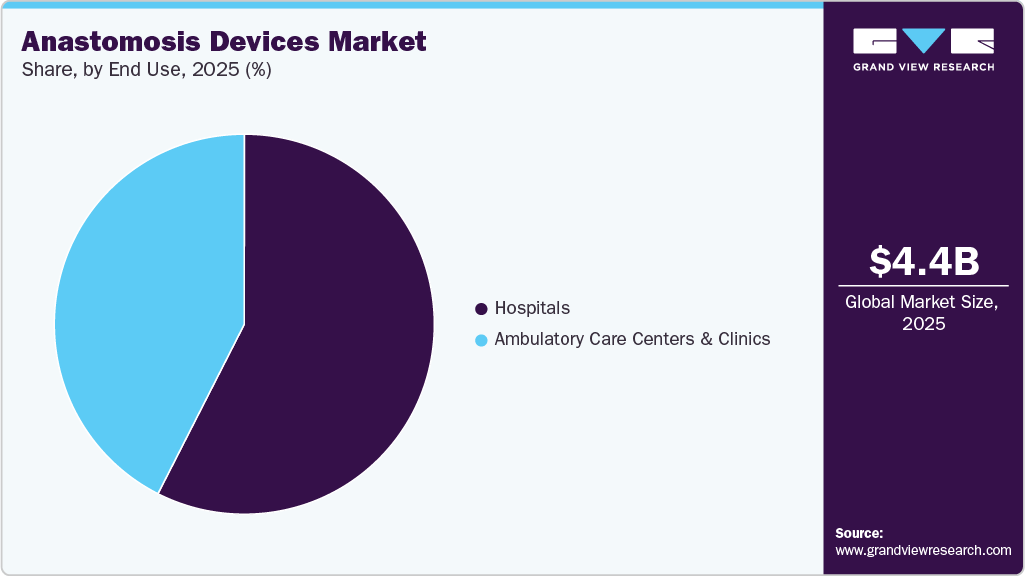
The ambulatory care centers & clinics segment is projected to grow at the fastest CAGR of 6.2% over the forecast period. The increasing demand for minimally invasive surgeries, which require shorter hospital stays and faster recovery times, the rising prevalence of chronic diseases, and the adoption of advanced surgical methods are driving market growth. Ambulatory care centers and clinics are well-equipped to handle surgical procedures and are therefore expected to see a surge in demand for their services.
Regional Insights
North America dominated the global anastomosis devices market with the largest revenue share of 40.1% in 2025. Market growth in the region is attributable to the high healthcare spending, the presence of well-established hospitals, and the significant presence of key players in the U.S. According to the National Center for Biotechnology Information (NCBI), in 2020, around 40 to 50 million surgeries were conducted in the U.S. This surge in surgical procedures is expected to increase the demand for anastomosis devices, propelling regional growth. Moreover, according to the Monmouth Medical Center (MMC), over 600,000 surgical procedures are performed in the U.S. annually to treat several colon diseases.
U.S. Anastomosis Devices Market Trends
The anastomosis devices market in the U.S. accounted for the largest market revenue share in North America in 2025. Rapid technological advancements and the presence of key manufacturers in the U.S. undertaking various initiatives, such as product approvals, product launches, mergers & acquisitions, to gain a competitive edge, are expected to contribute to market growth. According to the United Network for Organ Sharing, in January 2023, approximately 42,887 organ transplants were performed in the U.S. in 2022, representing a 3.7% increase over 2021.
Europe Anastomosis Devices Market Trends
The anastomosis devices market in Europe held a substantial market share in 2025, driven by the rising prevalence of chronic diseases, advanced healthcare infrastructure, and the adoption of minimally invasive surgical techniques. The region benefits from cutting-edge medical technologies and a high volume of surgical procedures, particularly in cardiovascular and gastrointestinal surgeries, which contribute to substantial market demand.
The Germany anastomosis devices market is expected to experience rapid growth over the forecast period. In 2016, Germany conducted approximately 51,000 heart bypass surgeries, driving demand for anastomosis devices. The country’s robust healthcare system and investment in innovative medical technologies are projected to sustain market growth, solidifying its position as a key market player.
Asia Pacific Anastomosis Devices Market Trends
The anastomosis devices market in Asia Pacific is expected to register at the fastest CAGR of 7.4% in the forecast period, fueled by increasing healthcare expenditures, improving medical infrastructure, and expanding awareness of advanced surgical techniques. Countries such as China and India are driving market growth, driven by an expanding patient population and rising demand for surgical interventions. The medical tourism industry is further boosting the adoption of anastomosis devices in the region.
The Japan anastomosis devices market is anticipated to grow at a significant CAGR during the forecast period. According to the latest data, 30% of Japan’s population is aged 65 or older, necessitating an increase in surgical needs. Advancements in healthcare technology and favorable reimbursement policies are facilitating the adoption of anastomosis devices in Japanese hospitals, supporting market growth and expansion.
Key Anastomosis Devices Company Insights
Some key companies operating in the market include Medtronic, MIZUHO Corporation, Getinge, Peters Surgical, and Baxter, among others. Key players operating in the market are focusing on technological innovations and developing advanced technology-based devices to gain a significant revenue share. Companies are focusing on Gripping Surface Technology (GST), magnetic anastomosis technology, and 3D stapling technology for developing anastomosis devices that can reduce leaks at the staple line and provide less invasive solutions for surgeons & patients.
-
MIZUHO Corporation specializes in advanced surgical instruments and medical devices tailored for anastomosis procedures. Their portfolio includes specialized clamps and suturing devices that enhance surgical precision and improve patient outcomes during complex cardiovascular surgeries.
-
Peters Surgical emphasizes innovative surgical solutions, notably their Enclose II Proximal Anastomosis Assist Device, designed for anastomosis during beating heart surgeries. Their products enhance efficiency and safety in surgical procedures, providing comprehensive solutions for diverse anastomotic techniques.
Key Anastomosis Devices Companies:
The following are the leading companies in the anastomosis devices market. These companies collectively hold the largest market share and dictate industry trends.
- Medtronic
- MIZUHO Corporation
- Getinge
- Peters Surgical
- Baxter
- Medical Device Business Services, Inc.
- CarpoNovum AB
- Vascular Graft Solutions Ltd.
- Medline Industries, LP.
- Seger Surgical Solutions Ltd.
Recent Developments
-
In May 2024, Ethicon announced the launch of the ECHELON LINEAR Cutter, the first surgical stapler combining 3D Stapling Technology and Gripping Surface Technology, significantly reducing staple line leaks and enhancing surgical outcomes.
-
In March 2024, Advanced Medical Solutions Group announced its proposed acquisition of Peters Surgical for approximately USD 141.4 million, enhancing its global position in tissue repair and skin closure technologies.
-
In March 2024, Getinge announced FDA 510(k) clearance for the Vasoview Hemopro 3, advancing endoscopic vessel harvesting technology with enhanced procedural efficiency and commitment to improving patient outcomes in cardiovascular surgery.
Anastomosis Devices Market Report Scope
Report Attribute
Details
Market size value in 2026
USD 4.66 billion
Revenue forecast in 2033
USD 7.07 billion
Growth rate
CAGR of 6.1% from 2026 to 2033
Base year for estimation
2025
Historical data
2021 - 2024
Forecast period
2026 - 2033
Quantitative units
Revenue in USD million/billion and CAGR from 2026 to 2033
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, trends
Segments covered
Product, application, end use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; Middle East & Africa
Country scope
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; UAE; Kuwait
Key companies profiled
Medtronic; MIZUHO Corporation; Getinge; Peters Surgical; Baxter; Medical Device Business Services, Inc.; CarpoNovum AB; Vascular Graft Solutions Ltd.; Medline Industries, LP.; Seger Surgical Solutions Ltd.
Customization scope
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope.
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Anastomosis Devices Market Report Segmentation
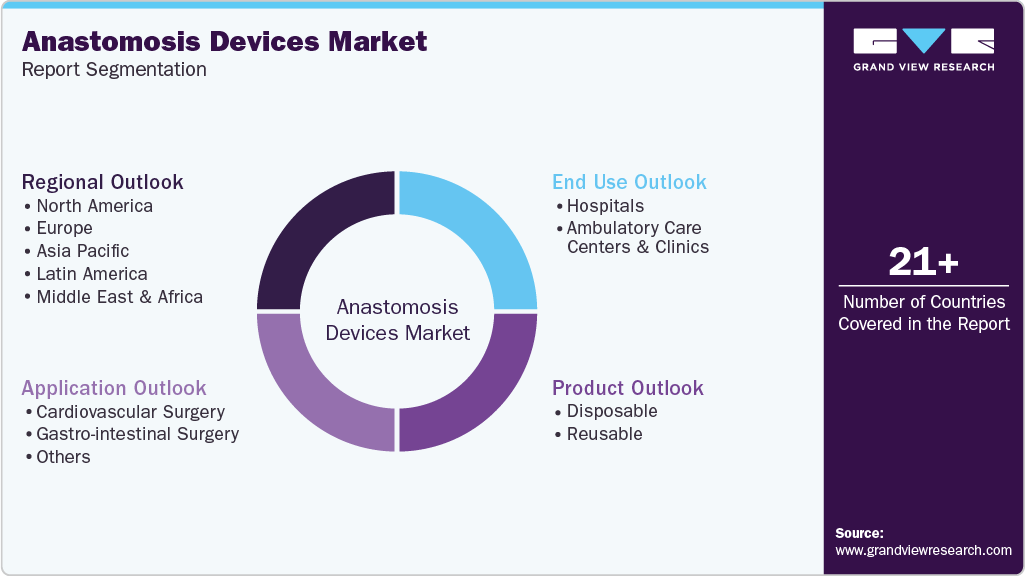
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Grand View Research has segmented the global anastomosis devices market report based on product, application, end use, and region:
-
Product Outlook (Revenue, USD Million, 2021 - 2033)
-
Disposable
-
Reusable
-
-
Application Outlook (Revenue, USD Million, 2021 - 2033)
-
Cardiovascular Surgery
-
Gastro-intestinal Surgery
-
Others
-
-
End Use Outlook (Revenue, USD Million, 2021 - 2033)
-
Hospitals
-
Ambulatory Care Centers & Clinics
-
-
Regional Outlook (Revenue, USD Million, 2021 - 2033)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Denmark
-
Sweden
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East & Africa
-
South Africa
-
Saudi Arabia
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global anastomosis devices market size was estimated at USD 4.41 billion in 2025 and is expected to reach USD 4.6 billion in 2026.
b. The global anastomosis devices market is expected to grow at a compound annual growth rate of 6.1% from 2026 to 2033 to reach USD 7.07 billion by 2033.
b. North America dominated the anastomosis devices market with a share of 40.1% in 2025. This is attributable to the increasing prevalence of cardiovascular diseases and a growing number of R&D collaborations carried out by major players in the region.
b. Some key players operating in the anastomosis devices market include Medtronic; LivaNova PLC; MAQUET Holding B.V. & Co. KG.; Dextera Surgical Inc.; Vitalitec Internaional Inc.; Synovis Micro Companies Alliance, Inc.; and Ethicon US, LLC., Peters Surgical.
b. Key factors that are driving the anastomosis devices market growth include rising surgical procedures along with the growing incidence of cardiovascular and gastrointestinal diseases, the introduction of technologically advanced products, and favorable reimbursement scenarios in cardiac procedures.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










