- Home
- »
- Clinical Diagnostics
- »
-
Alzheimer’s Disease Diagnostics Market Size Report, 2033GVR Report cover
![Alzheimer’s Disease Diagnostics Market Size, Share & Trends Report]()
Alzheimer’s Disease Diagnostics Market (2026 - 2033) Size, Share & Trends Analysis Report By Diagnostics Technique (Biomarkers, Imaging Techniques, Genetic Testing, Cognitive Assessment Tests), By Type (Triage, Diagnosis, Screening), By End User, By Region And Segment Forecasts
- Report ID: GVR-4-68040-323-7
- Number of Report Pages: 150
- Format: PDF
- Historical Range: 2021 - 2023
- Forecast Period: 2026 - 2033
- Industry: Healthcare
- Report Summary
- Table of Contents
- Interactive Charts
- Methodology
- Download FREE Sample
-
Download Sample Report
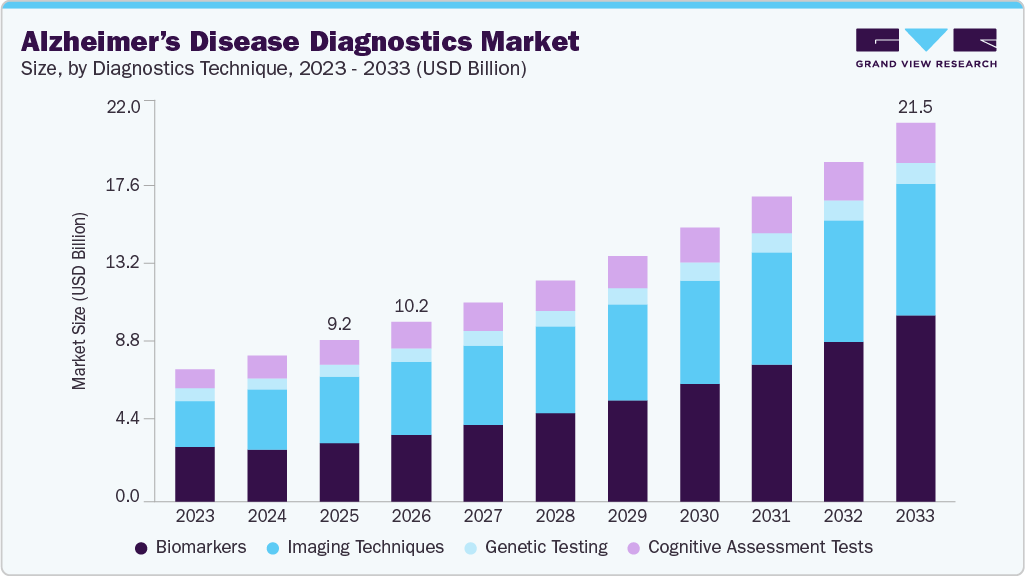
Alzheimer’s Disease Diagnostics Market Summary
TThe global Alzheimer's disease diagnostics market size was estimated at USD 9.22 billion in 2025 and is expected to reach USD 21.54 billion by 2033, projected to grow at a CAGR of 11.23% from 2026 to 2033. Increasing prevalence of Alzheimer’s Disease (AD), growing use of biomarkers in disease diagnostics, growing adoption of personalized products, and increasing technological advancements in medical imaging are some of the factors expected to drive the demand for Alzheimer’s diagnostics.
Key Market Trends & Insights
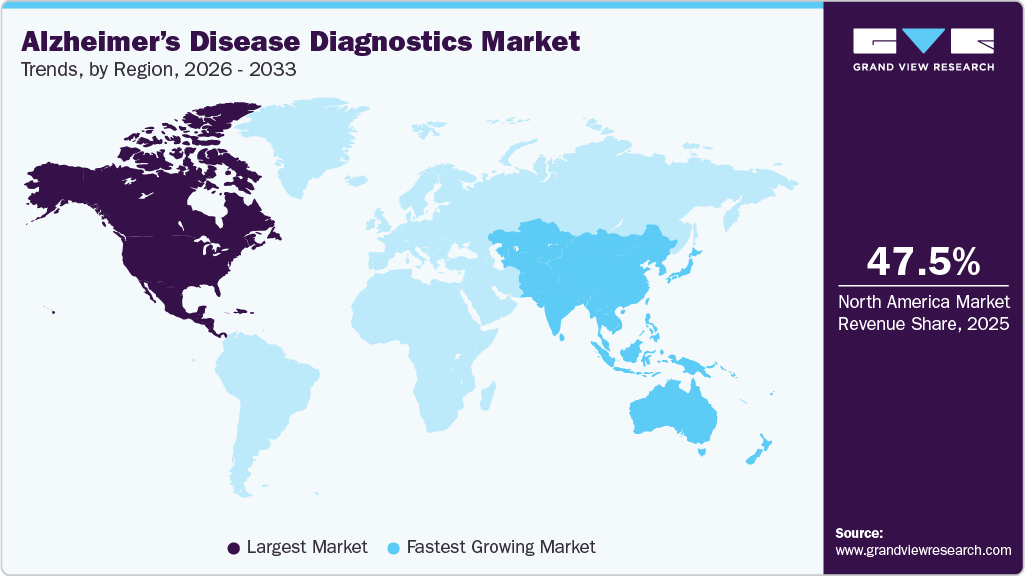
- North America Alzheimer's disease diagnostics market dominated the global market and accounted for the largest revenue share of 47.51% in 2025.
- The U.S. led the North American market and held the largest revenue share in 2025.
- Based on diagnostic technique, the imaging techniques segment held the largest revenue share of 40.80%in 2025.
- Based on type, the diagnosis segment dominated the global Alzheimer’s disease diagnostics market and diagnostics accounted for the largest revenue share of 54.09% in 2025.
- On the basis of end use, in academic and research institutes segment held the largest revenue share of 48.32% in 2025.
Market Size & Forecast
- 2025 Market Size: USD 9.22 Billion
- 2033 Projected Market Size: USD 21.54 Billion
- CAGR (2026-2033): 11.23%
- North America: Largest Market in 2025
- Asia Pacific: Fastest growing market
Increasing government investments and R&D studies is further propelling growth. The rising prevalence of Alzheimer's disease is a key driver of market growth. As the global population ages, the demand for early and accurate diagnostic tools continues to increase. According to the 2024 Alzheimer's Association, dementia risk rises with age, and the number of Americans with Alzheimer's is expected to grow significantly. By 2050, the population aged 65 and older is projected to reach 82 million, up from 58 million in 2022. By 2030, all baby boomers (born between 1946 and 1964) will be 65 or older, the age group most susceptible to Alzheimer's dementia.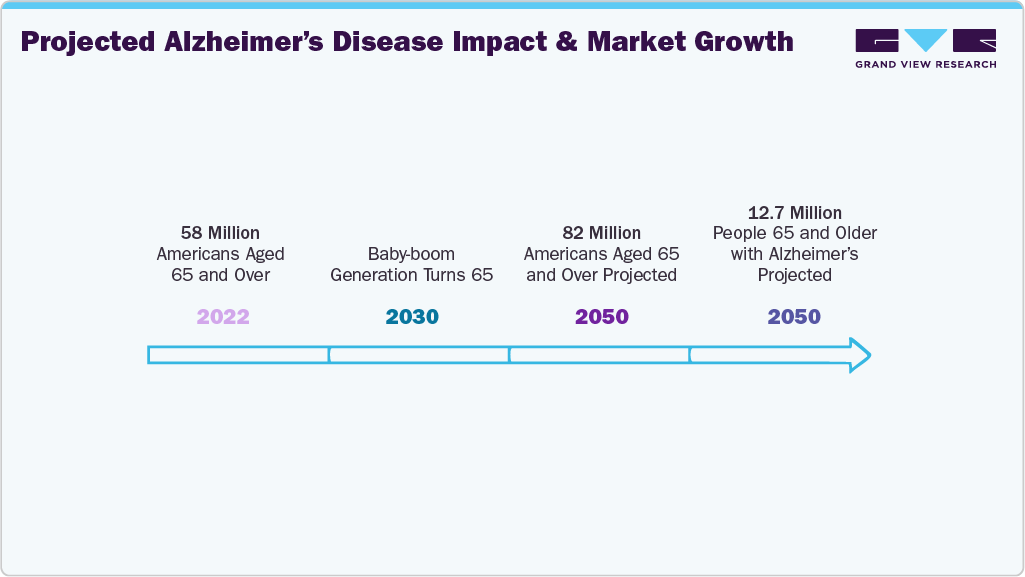
In recent years, there has been significant progress in developing novel therapeutic drugs targeting the root causes of Alzheimer’s disease. In January 2023, the U.S. FDA granted accelerated approval to lecanemab, a treatment co-developed by Eisai Co., Ltd. and Biogen Inc. Eisai also applied for manufacturing and marketing approval in Japan, where the drug received priority review from the Ministry of Health, Labour and Welfare (MHLW). However, effective use and distribution of these drugs require technology to detect amyloid-beta (Aβ) accumulation in the brain. Conventional testing methods, such as cerebrospinal fluid (CSF) testing and amyloid PET scans, are costly and invasive, increasing the demand for simpler, less invasive diagnostic approaches.
The growing global burden of Alzheimer’s has intensified the need for early detection and precise diagnosis. Advances in genomics, proteomics, and metabolomics have expanded the use of biomarkers in disease diagnostics. Biomarkers, particularly those detecting amyloid-beta and tau proteins in CSF through positron emission tomography (PET) imaging, play a crucial role in Alzheimer’s diagnosis. In addition, companies are launching innovative biomarker tests to support ongoing clinical trials. In March 2024, Labcorp introduced the pTau217 blood biomarker test, designed to accelerate Alzheimer’s diagnosis and improve clinical trial efficiency. This test is expected to enhance the accuracy and speed of detecting Alzheimer’s, ensuring timely treatment and disease management.
The rise of personalized medicine has underscored the importance of biomarkers in patient categorization, treatment selection, and therapeutic monitoring. This trend is expected to drive the biomarkers market’s expansion. A 2023 study published in the Journal of Alzheimer's Disease emphasized the role of precision medicine in Alzheimer’s, considering genetic, environmental, and lifestyle factors in disease progression. Similarly, an October 2021 study suggested bumetanide, an FDA-approved oral diuretic, as a potential treatment for Alzheimer’s. Research funded by the National Institute on Aging (NIA) analyzed brain tissue samples and FDA-approved drugs to explore repurposing opportunities, highlighting precision medicine’s potential in Alzheimer’s treatment.
Advancements in image analysis and machine learning algorithms have enhanced the accuracy and reliability of Alzheimer’s diagnostics through neuroimaging techniques. Diagnostic approaches have evolved from traditional cognitive assessments to advanced neuroimaging and CSF assays, allowing for early disease identification. In June 2023, Roche received FDA clearance for its CSF assay, which aids in timely diagnosis and treatment decision-making by measuring beta-amyloid and tau protein levels, two key biomarkers of Alzheimer’s pathology, in individuals aged 55 and older. These technological advancements are crucial for improving Alzheimer’s detection and management.
Ongoing research and technological innovations are driving the expansion of the Alzheimer’s diagnostics and therapeutics market. Companies continue to introduce new tests and products while engaging in strategic collaborations, mergers, and investments. A February 2024 MedPage Today article highlighted the diagnostic potential of blood biomarkers in Alzheimer’s research and clinical applications. Key industry players are advancing blood biomarker technologies to enhance global diagnostic capabilities. In August 2023, C2N Diagnostics launched the PrecivityAD2 blood test, designed to match the accuracy of PET scans and CSF tests. This test aims to assess patients with cognitive decline and Alzheimer’s symptoms, further strengthening diagnostic advancements in the field.
Market Concentration & Characteristics
The Alzheimer’s disease diagnostic market is characterized by a high degree of innovation, driven by advancements in diagnostic technologies such as biomarkers and neuroimaging techniques. These innovations enable more accurate and early diagnosis of Alzheimer’s, allowing for prompt intervention and individualized treatment. Key innovations include the use of blood-based and cerebrospinal fluid biomarkers combined with neuroimaging for improved diagnostic accuracy. For instance, in April 2024, Quest Diagnostics announced its portfolio expansion by adding a new blood biomarker test for phosphorylated tau 217 (p-tau217). Companies further have innovations in various blood-based biomarkers, cerebrospinal fluid tests, and genetic tests to provide a comprehensive offering in brain health. Such initiatives are expected to keep the degree of innovation high in the market over the forecast period.
The Alzheimer’s disease diagnostic market is characterized by the leading players with moderate levels of technology launches and merger and acquisition (M&A) activity. Market players like Quest and others are involved in new product launches and merger and acquisition activities. For instance, in February 2023, Lantheus announced the acquisition of Cerveau Technologies, a company focused on developing imaging agents for Alzheimer's Disease research and clinical trials. This acquisition aligns with Lantheus' growth strategy and provides the potential to utilize MK-6240 (an F-18 labeled PET imaging agent that targets tau tangles in Alzheimer's Disease) as a key clinical tool supporting patient care as more Alzheimer's treatments.
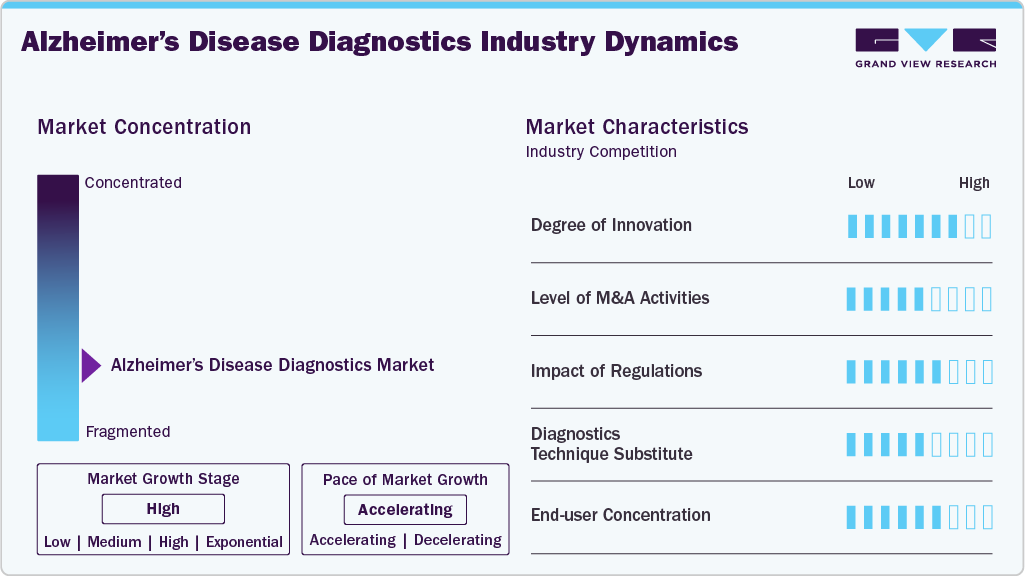
Regulation has a significant impact on the Alzheimer's Diagnostic market. The increasing number of regulatory criteria and approvals changes for Alzheimer's diagnostics products underscores the need for reliable tests and contributes to market growth. For instance, in July 2024, an article by Alzheimer’s Association highlighted discussions around the revised diagnostic criteria for Alzheimer’s disease and its staging. The criteria were developed by the Alzheimer's Association and the National Institute on Aging (NIA) and aim to improve the diagnosis and characterization of Alzheimer’s. The revised criteria refine and broaden previous guidelines issued in 2011 and 2018. They incorporate new scientific insights and technological advances to improve current diagnosis and establish a research agenda for future progress.
There is no direct substitute to existing disease diagnostic products and treatments, as various diagnostic tests and tools serve complementary roles in assessing risk, aiding diagnosis, and monitoring disease progression. However, there are alternatives that can provide valuable information, such as cognitive assessments, neurological exams, genetic testing, spinal fluid analysis, and neuropsychological tests. These alternatives are often used in conjunction with biomarker tests to provide a comprehensive understanding of an individual's cognitive health.
The market mostly comprises of end users as hospital and clinicians allowing hospitals and clinics to purchase the necessary equipment and drugs needed to treat patients with Alzheimer’s. This concentration of end users in institutional settings drives the demand for reliable and accurate diagnostic tests, which in turn fuels the growth of the market.
Case Study: Diagnostic Insights from a Novel PSEN2 Mutation in Alzheimer’s Disease
A 63-year-old woman was diagnosed with early-onset Alzheimer’s disease (EOAD), confirmed postmortem through histopathology. Initial symptoms included memory loss, executive dysfunction, and visuospatial deficits, progressing to total dependence over six years. Neuroimaging revealed cortical and hippocampal atrophy. Genetic testing identified a novel PSEN2 mutation (L221T) and an ApoE3/4 genotype, suggesting a pathogenic link. The mutation likely altered γ-secretase activity, increasing amyloid-β42 deposition. Autopsy findings confirmed extensive amyloid plaques, tau tangles, and Lewy body pathology. This case underscores the importance of genetic screening in atypical Alzheimer’s presentations, particularly in patients with no clear familial history. Advances in biomarkers and neuroimaging remain crucial for early and accurate diagnosis..
Diagnostics Technique Insights
The imaging technique segment accounted for the largest revenue share of 40.80% in 2025, driven by the critical need for early and accurate disease detection. Advances in imaging technologies, including Computed Tomography (CT), Positron Emission Tomography (PET), and functional MRI (fMRI), have become essential tools for diagnosing and monitoring Alzheimer’s disease. These methods help identify key disease-related changes, such as amyloid plaque buildup and brain atrophy. Companies are actively seeking market approval for innovative medical imaging solutions. For example, in November 2023, Pixyl, a French medtech company, received FDA 510(k) clearance for its AI-powered brain MRI software, Pixyl.Neuro. This software leverages generative AI to analyze brain MRI scans, enabling faster detection, early diagnosis, and objective monitoring of neurological disorders. Such advancements are expected to sustain the strong market share of imaging techniques in the coming years.
The biomarkers segment is expected to witness the fastest growth over the forecast period, driven by the increasing demand for biomarkers to diagnose and monitor Alzheimer’s. This segment is further categorized into CSF biomarkers and blood-based biomarkers. CSF biomarkers, including amyloid-beta (Aβ), tau, and neurofilament light chain (NfL), are widely used in clinical practice to aid in diagnosing Alzheimer’s and other dementias. Meanwhile, blood-based biomarkers are being actively researched for their potential in detecting Alzheimer’s through non-invasive blood tests. Among biomarkers, blood-based biomarkers are projected to experience the most rapid growth due to recent breakthroughs that enhance early detection, diagnosis, and disease monitoring. In July 2023, ALZpath, a U.S.-based leader in Alzheimer’s diagnostic solutions, secured funding from the Alzheimer’s Drug Discovery Foundation (ADDF) to accelerate the clinical launch of ALZpathDx-a laboratory-developed test that uses a novel blood-based biomarker to detect Alzheimer’s with unprecedented accuracy. This investment aims to fast-track the commercialization of ALZpathDx, empowering healthcare professionals with better diagnostic tools and improving patient outcomes.
In addition, new developments in blood biomarkers are expected in 2024. In February 2024, MedPage Today reported that discussions on the clinical use of blood biomarkers will take place at the 2024 Alzheimer’s Association International Conference (AAIC) in July. The conference will also see the release of new guidelines and a systematic review on the subject, further driving innovation and adoption of biomarker-based diagnostics. These advancements highlight the growing importance of blood biomarkers in Alzheimer’s research and clinical practice.
Type Insights
The diagnostic segment dominated the market in 2025, holding the largest share at 54.09% in 2025. The growing global market is driven by an aging population, increasing the number of individuals at risk for Alzheimer's disease and fueling demand for early and accurate diagnostic tools. Technological advancements, such as LMI and SOFIE’s Neuraceq PET imaging agent, as well as the development of cerebrospinal fluid (CSF) biomarkers and blood-based tests, are significantly improving diagnostic precision and accessibility. These innovations are critical for early intervention and better patient outcomes.
Awareness initiatives also play a key role in market expansion. Organizations such as the Alzheimer’s Association and the World Health Organization (WHO) are actively promoting the importance of early diagnosis through educational programs, public service campaigns, and community outreach, encouraging individuals to seek assessments at the first signs of cognitive decline.
Furthermore, government policies are creating new growth opportunities. In October 2023, U.S. health officials removed restrictions on the reimbursement of amyloid PET scans, a non-invasive imaging test used in Alzheimer’s diagnosis. Previously limited to a once-per-lifetime use, this change enables wider access to the test, aiding in the identification of candidates for emerging treatments. These include Eisai and Biogen’s Leqembi and Eli Lilly’s investigational drug, donanemab, both designed to target and clear beta-amyloid protein from the brain. Expanded access to amyloid PET scans is expected to improve early and accurate diagnosis, which is essential for determining eligibility for these FDA-approved therapies. Amyloid confirmation is also required for government reimbursement of Leqembi and similar treatments through a national data collection registry managed by the Centers for Medicare & Medicaid Services (CMS).
End Use Insights
In 2025, the academic and research institutes segment led the end-use market, holding the largest share at 48.32%. This dominance is driven by the increasing number of research studies and clinical trials focused on neurological disorders over the past decade. Academic and research institutions play a pivotal role in advancing research, conducting clinical trials, and developing innovative diagnostic techniques. These institutions often receive significant funding from government agencies, private foundations, and industry partners. For example, the National Institute on Aging (NIA) funds Alzheimer’s Disease Research Centers (ADRCs) at major medical institutions across the U.S., supporting extensive research efforts.
Institutions are actively engaged in evaluating new diagnostic tools. For instance, the Alzheimer’s Disease Research Center at the Icahn School of Medicine at Mount Sinai is utilizing blood-based biomarkers for Alzheimer’s diagnosis. In addition, studies such as the AHEAD 3-45 trial of lecanemab (Leqembi) and the TRAILBLAZER-ALZ trial of investigational donanemab use blood markers to identify suitable patients for treatment.
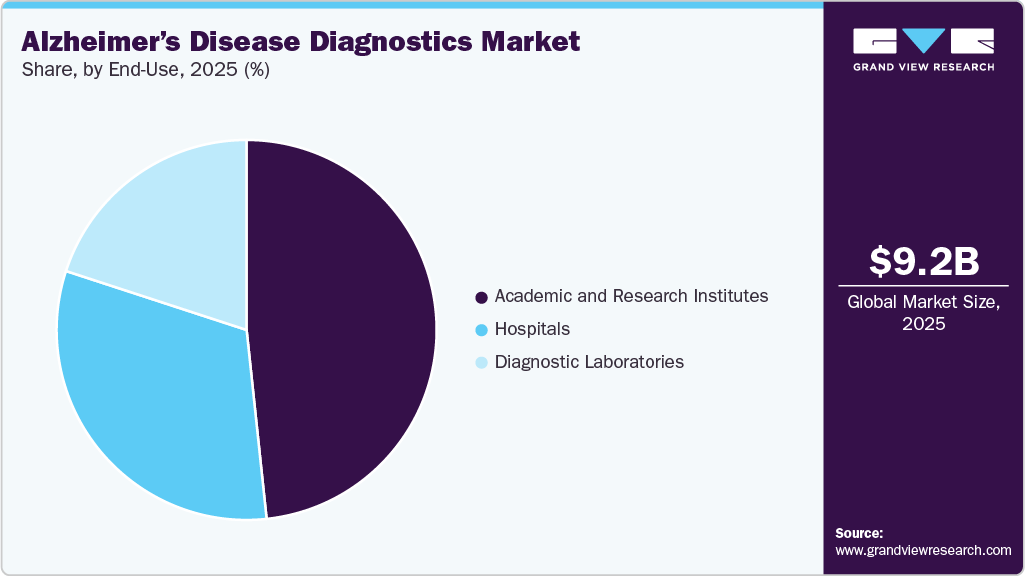
The hospitals segment is also anticipated to grow at a faster pace, driven by the increasing emphasis on early detection and diagnosis of Alzheimer’s disease. Hospitals serve as primary healthcare settings where patients seek treatment and care, and they are equipped with advanced diagnostic technologies, including neuroimaging techniques such as MRI and PET scans, as well as biomarker tests. These cutting-edge tools are essential for accurate and early diagnosis, facilitating timely intervention and improved patient management. In addition, hospitals are integrating blood-based biomarkers into their diagnostic protocols to enhance patient care.
Regional Insights
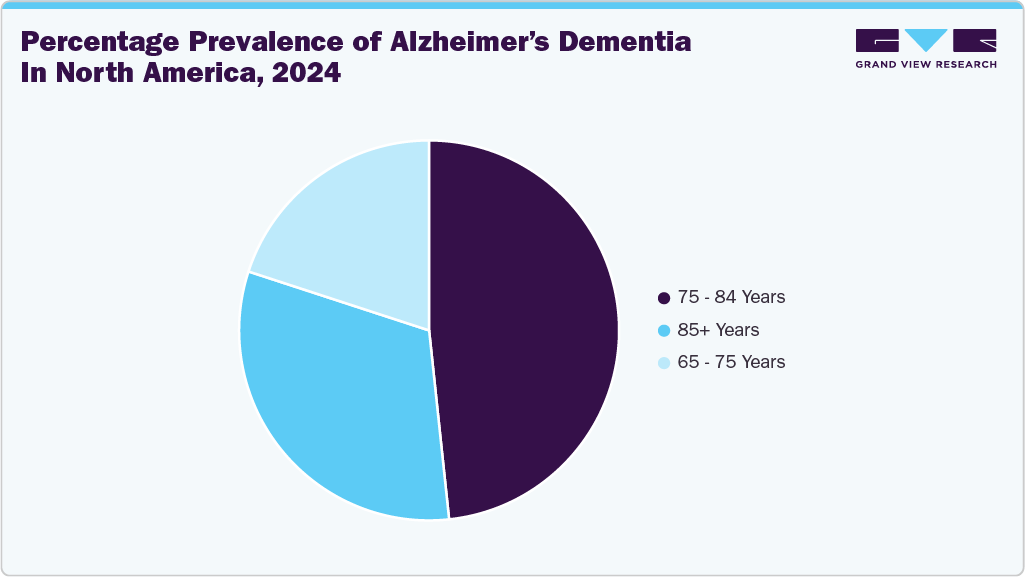
The Alzheimer's disease diagnostics market in North America held the largest share of 47.51% in 2025. This significant market presence is attributed to the presence of key industry players such as LabCorp, Quanterix, and Sysmex, among others. In addition, the market growth in North America is primarily driven by the rising prevalence of Alzheimer’s disease. Currently, approximately 6.7 million Americans aged 65 and older are living with Alzheimer’s dementia, a number projected to reach 13.8 million by 2060 unless medical advancements are made to prevent, slow, or cure the disease. The Alzheimer Society of Canada reported that there are 771,939 individuals in Canada affected by dementia as of January 2025. Experts predict that by 2030, over one million Canadians will have dementia, and projections indicate that by 2050, over 1.7 million people in Canada will be dealing with dementia.
The region is also leading in Alzheimer’s disease biomarker research, including cerebrospinal fluid analysis and blood-based biomarkers. These advancements are enabling the development of more accurate and minimally invasive diagnostic tests, significantly contributing to market expansion.
U.S. Alzheimer’s Disease Diagnostics Market Trends
The Alzheimer’s disease diagnostics market in the U.S. is poised for substantial growth during the forecast period. This is largely due to significant investments in research and development (R&D) by government agencies and nonprofit organizations such as the Alzheimer’s Drug Discovery Foundation (ADDF). The emergence of digital biomarkers and advancements in diagnostic technologies further support market expansion.
Academic institutions, pharmaceutical companies, and government agencies in the U.S. are actively collaborating to develop and validate new diagnostic tools and biomarkers for Alzheimer’s disease. For example, in July 2023, Quest Diagnostics introduced the AD-Detect Test, a revolutionary blood-based biomarker test available to consumers in the U.S. This innovative test helps individuals assess their risk of developing Alzheimer’s by measuring beta-amyloid protein levels, a key indicator of the disease.
Europe Alzheimer’s Disease Diagnostics Market Trends
Europe Alzheimer’s disease diagnostics market has been identified as a promising region, with growing awareness among healthcare professionals & patients and rising prevalence fueling market growth. In January 2025, the European Brain Council reported that approximately 7 million people in Europe are currently affected by Alzheimer's disease. With an aging population, this issue poses a serious public health challenge internationally, with an estimated 14 million by 2030. The European market is highly competitive, featuring multiple key players such as Eli Lilly and Company, Bio-Rad Laboratories, and Quest Diagnostics, among others. These companies are focusing on developing more accurate and reliable tests while expanding their geographical presence.
The Alzheimer’s disease diagnostics market in the UK is expected to grow steadily due to increased government efforts and significant investments in developing new clinical tools for dementia and neurodegenerative diseases. In March 2024, UK Research and Innovation announced that Innovate UK awarded $6.24 million in funding through the Small Business Research Initiative (SBRI) to support the development of improved Alzheimer’s disease diagnostic technologies. These initiatives are anticipated to accelerate the adoption of novel biomarkers, AI-based screening technologies, and enhanced neuroimaging methods into conventional clinical practice. Collaborations between academic institutions, biotech corporations, and healthcare providers are also helping to advance the development of early detection systems. Adoption rates in primary care settings are also projected to be increasing as public health campaigns encouraging rapid diagnoses become more prevalent
Germany’s Alzheimer’s disease diagnostics market is projected to expand in response to the country’s aging population. With a significant rise in the elderly demographic, the demand for Alzheimer’s diagnostics is expected to increase, particularly among older adults who are more prone to neurological conditions like dementia and Alzheimer’s. By 2040, nearly 21.4 million Germans are expected to be in this high-risk age group, underscoring the growing need for advanced diagnostic solutions. Increasing focus of leading players on technologically advanced product launches is a positive growth factor. For instance, in October 2024, Siemens Healthineers (Germany) received CE Mark for two new syngo functionalities. PET Cortical Analysis software uses positron emission tomography (PET) to detect protein accumulations in the brain associated with Alzheimer's disease. The first new feature, Centiloid scoring, standardizes the measurement of brain amyloid plaques in PET scans using three commercially available beta-amyloid PET radiopharmaceuticals used to diagnose Alzheimer's disease. The second new feature, tau PET quantification, assesses and quantifies the location and density of tau protein tangles in the brain using a PET scan produced with the radiopharmaceutical flortaucipir.
Asia Pacific Alzheimer’s Disease Diagnostics Market Trends
The Asia-Pacific Alzheimer’s disease diagnostics market is expected to experience the fastest growth, with a projected CAGR of 12.96% over the forecast period. This rapid expansion is attributed to rising public awareness and increased research efforts aimed at developing new treatments for Alzheimer’s. Many countries in the region, including China, Japan, and India, are witnessing a demographic shift toward an aging population, which is more susceptible to age-related diseases such as Alzheimer’s. This growing elderly population is fueling demand for accurate and early diagnostic services. In addition, increasing investments in new testing technologies and product development are expected to create favorable market opportunities in the region.
China’s Alzheimer’s disease diagnostics market is anticipated to grow significantly, driven by the country’s active involvement in Alzheimer’s research, including the development and validation of new diagnostic methods. For instance, in August 2025, BGI Genomics developed its own blood-based test for Alzheimer's disease and other cognitive disorders after receiving China's national regulatory approval in April 2024. This test looks for five key signs in the blood, including specific forms of tau and amyloid proteins, along with other nerve-related indicators, that together give doctors a clearer picture of what’s happening in the brain. Ongoing clinical trials for investigational diagnostic tools and biomarkers are further propelling market growth.
Japan’s Alzheimer’s disease diagnostics market is set for expansion due to the rising prevalence of dementia. The number of dementia patients in Japan is estimated to reach 6-7 million by 2025, heightening the demand for early diagnosis and treatment of Alzheimer’s, which accounts for 60%-70% of all dementia cases.
Manufacturers in Japan are actively developing new assay kits to address Alzheimer’s pathology. For example, in June 2023, Sysmex Corporation launched the HISCL β-Amyloid 1-42 and 1-40 Assay Kit in Japan. These kits measure amyloid beta (Aβ) levels in the blood, assisting in the identification of Aβ accumulation in the brain-a hallmark of Alzheimer’s disease..
Latin America Alzheimer’s Disease Diagnostics Market Trends
The Latin American Alzheimer’s disease diagnostics market is expected to grow significantly over the forecast period. The region has a rapidly aging population, with a high percentage of individuals over 65-an age group at increased risk for Alzheimer’s. The demand for personalized medicine and precision diagnostics is also on the rise. Moreover, collaborations between pharmaceutical and diagnostic companies in countries such as Brazil and Argentina are fostering the development of minimally invasive diagnostic solutions.
Brazil’s Alzheimer’s disease diagnostics market is projected to expand due to government initiatives aimed at enhancing healthcare infrastructure and increasing access to diagnostic services, particularly in rural areas. In addition, the presence of both global and local market players is supporting industry growth. However, challenges such as the need for more accurate diagnostic tests, high treatment costs, and limited healthcare access in some regions persist.
Middle East & Africa Alzheimer’s Disease Diagnostics Market Trends
The Middle East and Africa Alzheimer’s disease diagnostics industry is gradually gaining momentum, driven by increasing awareness of critical health issue and the growing need for effective diagnostic solutions. The region's high rate of hospital-acquired infections and growing burden of infectious diseases are both major factors driving the need for improved diagnostics. Moreover, in April 2025, the National Reference Laboratory (NRL) center in Abu Dhabi opened in collaboration with Neurocode International, becoming the first dedicated facility in the region to provide early Alzheimer's detection via a simple blood test, targeting individuals over the age of 40. These initiatives are part of a bigger movement in the MEA region to promote early identification and preventative healthcare. As access to less intrusive diagnostic methods increases, the market is expected to grow faster, potentially improving patient outcomes, reducing long-term care issues, and providing the groundwork for comprehensive dementia care in the region.
Saudi Arabia’s Alzheimer’s disease diagnostics market is expected to experience growth in the coming years, supported by substantial government investments in healthcare modernization. The country is actively establishing advanced diagnostic facilities and specialized centers for neurological disorders. These initiatives are creating opportunities for the adoption of cutting-edge Alzheimer’s diagnostic tools, including blood-based biomarkers.
Key Alzheimer’s Disease Diagnostics Company Insights
Some of the key players operating in the market include Quest Diagnostics Incorporated, Abbott, F. Hoffmann-La Roche Ltd., Eli Lily, Bio-Rad Laboratories, Inc, Eisai Co Ltd and others. The market is highly competitive, with a large number of manufacturers accounting for a majority of the share. New source developments, mergers and acquisitions, and collaborations are some of the major strategies adopted by these players to counter the stiff competition.
Key Alzheimer’s Disease Diagnostics Companies:
The following are the leading companies in the alzheimer’s disease diagnostics market. These companies collectively hold the largest market share and dictate industry trends.
- Quest Diagnostics
- Labcorb
- C2N diagnostics
- FujireBio
- Hoffmann-La Roche
- Quanterix
- Sysmex
- Lantheus
- Siemens Healthineers
Recent Developments
-
In August 2025, C2N Diagnostics launched the PrecivityAD2 blood tests, targeting those over the age of 60. The company just completed additional validation to extend eligibility to those aged 50 and up, which covers anyone born in 1975 and earlier. The PrecivityAD2 blood test can help clinicians diagnose early-onset Alzheimer's disease in people aged 50 to 55. The test helps healthcare personnel discover amyloid plaques in the brain, which are a key sign of Alzheimer's disease, and also informs medical management and treatment decisions
-
In May 2025, the FDA approved the first in vitro diagnostic equipment designed for analyzing blood to assist in the diagnosis of Alzheimer's disease. The Lumipulse G pTau217 /ß-Amyloid 1-42 Plasma Ratio is intended to detect amyloid plaques associated with Alzheimer's disease in adults aged 55 and older who demonstrate symptoms of the condition.
-
In January 2025, Lantheus Holdings, Inc. announced an agreement to acquire Life Molecular Imaging in an all-cash transaction. This acquisition aims to enhance diagnostic solutions for patients in the expanding Alzheimer's disease radiodiagnostic market.
-
In January 2025, The Global Alzheimer’s Platform Foundation (GAP) announced the addition of Beckman Coulter as a strategic partner in the Bio-Hermes-002 study. This inaugural collaboration will strengthen the unique observational platform study, which compares blood-based and digital biomarkers alongside clinical cognitive conditions, MRI and PET images, and a diverse range of races and ethnicities. The aim is to generate data that could aid in predicting, detecting, and diagnosing Alzheimer’s disease and related dementias.
-
In January 2025,, Beckman Coulter announced that the FDA granted Breakthrough Device Designation to the p‑Tau217/β-Amyloid plasma ratio test. This blood test is designed to assist healthcare providers in identifying patients with amyloid pathology linked to Alzheimer's disease
Alzheimer’s Disease Diagnostics Market Report Scope
Report Attribute
Details
Market size value in 2026
USD 10.22 billion
Revenue forecast in 2033
USD 21.54 billion
Growth rate
CAGR of 11.23% from 2026 to 2033
Actual data
2021 - 2023
Forecast period
2026 - 2033
Quantitative units
Revenue in USD million/billion and CAGR from 2026 to 2033
Report coverage
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
Segments covered
Diagnostics technique, type, end use, region
Regional scope
North America; Europe; Asia Pacific; Latin America; MEA
Country scope
U.S.; Canada; Mexico; UK; Germany; France; Italy; Spain; Sweden; Denmark; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Argentina; Saudi Arabia; South Africa; UAE; Kuwait
Key companies profiled
Quest Diagnostics; Labcorp; C2N Diagnostics; Fujirebio; Hoffmann-La Roche; Quanterix; Sysmex; Lantheus; Siemens Healthineers
Customization scope
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope
Pricing and purchase options
Avail customized purchase options to meet your exact research needs. Explore purchase options
Global Alzheimer’s Disease Diagnostics Market Segmentation
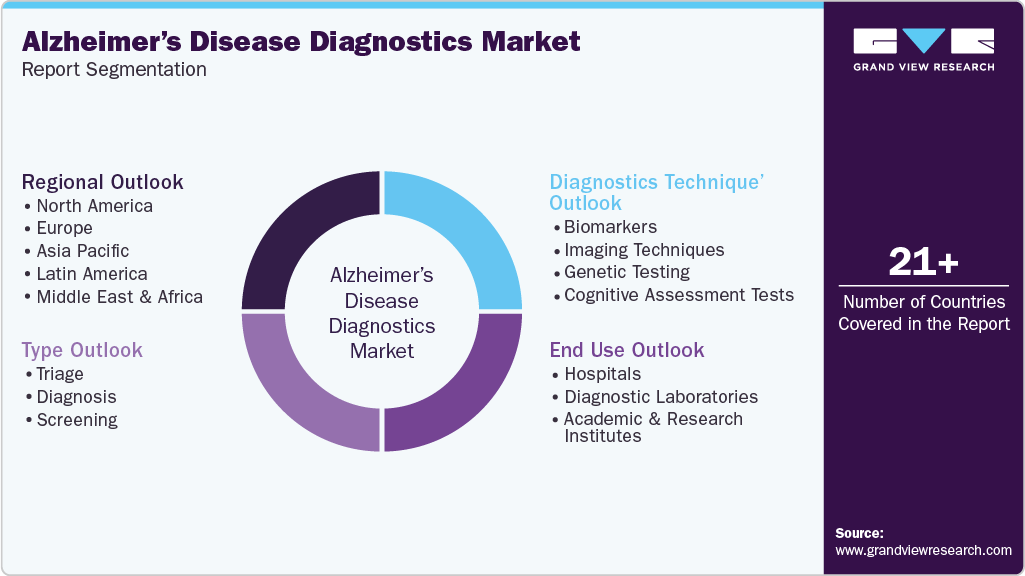
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For the purpose of this study, Grand View Research has segmented the global Alzheimer’s disease diagnostics market report on the basis of diagnostics technique, type, end use, and region.
-
Diagnostics Technique Outlook (Revenue, USD Million, 2021 - 2033)
-
Biomarkers
-
CSF Biomarkers
-
Blood-Based Biomarkers
-
-
Imaging Techniques
-
Genetic Testing
-
Cognitive Assessment Tests
-
-
Type Outlook (Revenue, USD Million, 2021 - 2033)
-
Triage
-
Diagnosis
-
Screening
-
-
End Use Outlook (Revenue, USD Million, 2021 - 2033)
-
Hospitals
-
Diagnostic Laboratories
-
Academic and Research Institutes
-
-
Regional Outlook (Revenue in USD Million, 2021 - 2033)
-
North America
-
U.S.
-
Canada
-
Mexico
-
-
Europe
-
UK
-
Germany
-
France
-
Italy
-
Spain
-
Sweden
-
Denmark
-
Norway
-
-
Asia Pacific
-
Japan
-
China
-
India
-
Australia
-
South Korea
-
Thailand
-
-
Latin America
-
Brazil
-
Argentina
-
-
Middle East and Africa
-
Saudi Arabia
-
South Africa
-
UAE
-
Kuwait
-
-
Frequently Asked Questions About This Report
b. The global Alzheimer’s disease diagnostics market size was estimated at USD 9.22 billion in 2025 and is expected to reach USD 10.22 billion in 2026.
b. The global Alzheimer’s disease diagnostics market is expected to grow at a compound annual growth rate of 11.23% from 2026 to 2033, reaching USD 21.54 billion by 2033.
b. The Alzheimer's disease diagnostics market in North America held the largest share of 47.51% in 2025. This is attributable to the increasing prevalence of alzheimer’s in the region
b. Some key players operating in the Alzheimer’s disease diagnostics market include Quest Diagnostics; Labcorp; C2N Diagnostics; Fujirebio; Hoffmann-La Roche; Quanterix; Sysmex; Lantheus; Siemens Healthineers
b. Key factors driving market growth are the increasing prevalence of Alzheimer’s disease (AD), the growing use of biomarkers in disease diagnostics, the growing adoption of personalized products, and the increasing technological advancements in medical imaging.
Share this report with your colleague or friend.
Need a Tailored Report?
Customize this report to your needs — add regions, segments, or data points, with 20% free customization.

ISO 9001:2015 & 27001:2022 Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
Trusted market insights - try a free sample
See how our reports are structured and why industry leaders rely on Grand View Research. Get a free sample or ask us to tailor this report to your needs.










