India Pulmonary Arterial Hypertension Market Size & Outlook
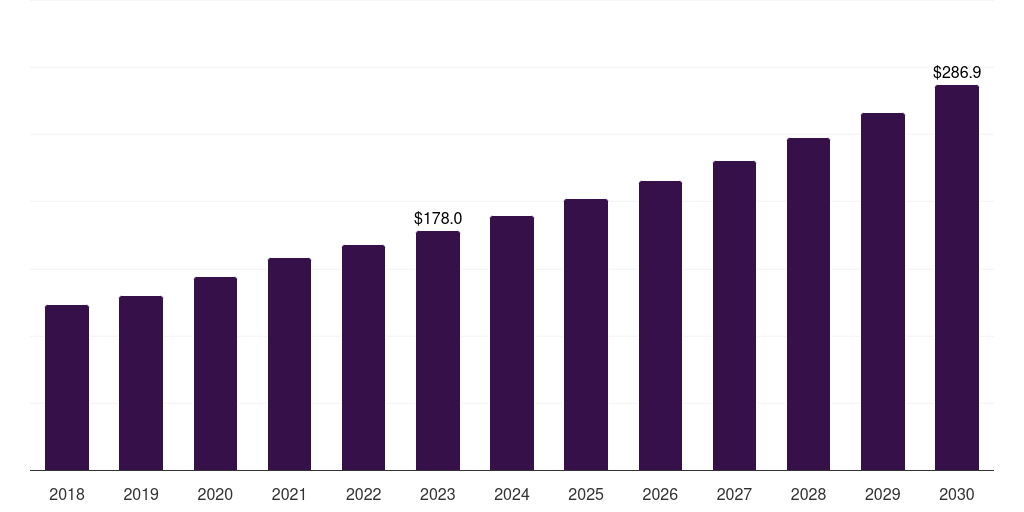
India pulmonary arterial hypertension market, 2018-2030 (US$M)
Related Markets
India pulmonary arterial hypertension market highlights
- The India pulmonary arterial hypertension market generated a revenue of USD 178.0 million in 2023 and is expected to reach USD 286.9 million by 2030.
- The India market is expected to grow at a CAGR of 7.1% from 2024 to 2030.
- In terms of segment, prostacyclin and prostacyclin analogs was the largest revenue generating drug class in 2023.
- SGC Stimulators is the most lucrative drug class segment registering the fastest growth during the forecast period.
Pulmonary arterial hypertension market data book summary
| Market revenue in 2023 | USD 178.0 million |
| Market revenue in 2030 | USD 286.9 million |
| Growth rate | 7.1% (CAGR from 2023 to 2030) |
| Largest segment | Prostacyclin and prostacyclin analogs |
| Fastest growing segment | SGC Stimulators |
| Historical data | 2018 - 2022 |
| Base year | 2023 |
| Forecast period | 2024 - 2030 |
| Quantitative units | Revenue in USD million |
| Market segmentation | Endothelin Receptor Antagonists (ERAs), PDE-5 Inhibitors, Prostacyclin and Prostacyclin Analogs, SGC Stimulators |
| Key market players worldwide | United Therapeutics Corp, Bayer AG, Gilead Sciences Inc, Johnson & Johnson, Viatris Inc, GlaxoSmithKline, Sandoz Group AG ADR, Lupin, Sun Pharmaceutical Industries, Teva Pharmaceutical Industries Ltd |
Other key industry trends
- In terms of revenue, India accounted for 2.4% of the global pulmonary arterial hypertension market in 2023.
- Country-wise, U.S. is expected to lead the global market in terms of revenue in 2030.
- In Asia Pacific, Japan pulmonary arterial hypertension market is projected to lead the regional market in terms of revenue in 2030.
- South Korea is the fastest growing regional market in Asia Pacific and is projected to reach USD 191.4 million by 2030.
Prostacyclin and prostacyclin analogs was the largest segment with a revenue share of 47.13% in 2023. Horizon Databook has segmented the India pulmonary arterial hypertension market based on endothelin receptor antagonists (eras), pde-5 inhibitors, prostacyclin and prostacyclin analogs, sgc stimulators covering the revenue growth of each sub-segment from 2018 to 2030.
According to WHO report, 2016, the incidence of PAH ranges from one, two cases per million in general population. The incidence of disorder in HIV infection or portal hypertension has been estimated to be 0.5% to 2%. Various treatment options ranging from calcium channel blockers, and local treatment methods such as Ayurveda are available in the country. However, newer agents show a great promise in the treatment of PAH.
Key strategies are undertaken by market players to strengthen their foothold in the PAH market, which include new product developments. For instance, in April 2019, Cipla received final approval from the U.S. FDA for Ambrisentan tablets, specified for the treatment of PAH. In September 2020, Alembic Pharmaceuticals received tentative approval from the U.S. health regulator for Treprostinil injection, designated for the treatment of PAH.
Organization for Rare Diseases India (ORDI) is a national umbrella organization demonstrating the collective voice of all patients with rare diseases in the country. The government proposed financial support of up to ₹1,500,000 under the Rashtriya Arogaya Nidhi, for those that require a one-time treatment under the Rare Diseases Policy 2020. Such government policies are expected to promote market growth during the forecast period.
No credit card required*
Horizon in a snapshot
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more
Pulmonary Arterial Hypertension Market Scope
Pulmonary Arterial Hypertension Market Companies
| Name | Profile | # Employees | HQ | Website |
|---|
India pulmonary arterial hypertension market size, by drug class, 2018-2030 (US$M)
India Pulmonary Arterial Hypertension Market Outlook Share, 2023 & 2030 (US$M)
Related regional statistics
No records
No related regions found.
Sign up - it's easy, and free!
Sign up and get instant basic access to databook, upgrade
when ready, or enjoy our
free plan indefinitely.
Included in Horizon account
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more


