Mexico Crispr And Cas Genes Market Size & Outlook
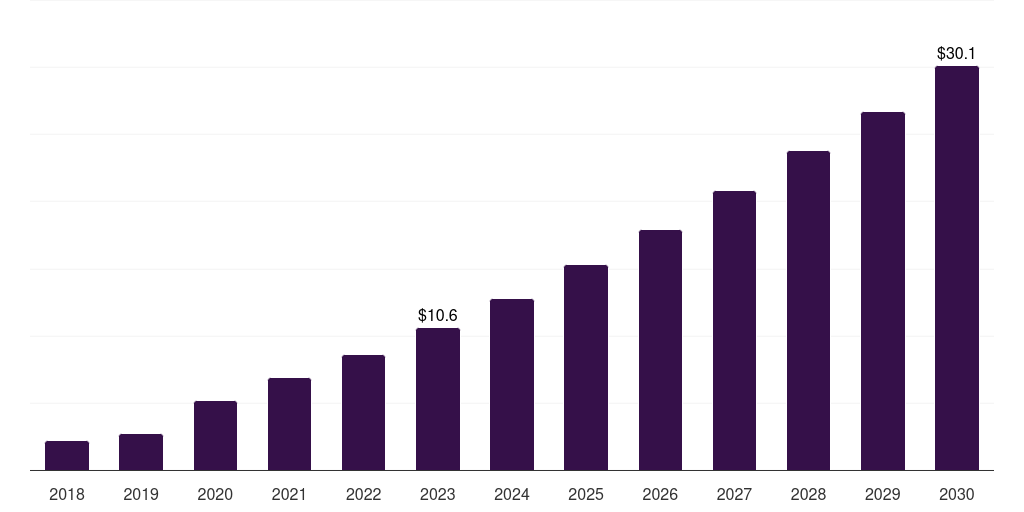
Mexico crispr and cas genes market, 2018-2030 (US$M)
Related Markets
Mexico crispr and cas genes market highlights
- The Mexico crispr and cas genes market generated a revenue of USD 10.6 million in 2023 and is expected to reach USD 30.1 million by 2030.
- The Mexico market is expected to grow at a CAGR of 16.2% from 2024 to 2030.
- In terms of segment, product was the largest revenue generating product & service in 2023.
- Service is the most lucrative product & service segment registering the fastest growth during the forecast period.
Crispr and cas genes market data book summary
| Market revenue in 2023 | USD 10.6 million |
| Market revenue in 2030 | USD 30.1 million |
| Growth rate | 16.2% (CAGR from 2023 to 2030) |
| Largest segment | Product |
| Fastest growing segment | Service |
| Historical data | 2018 - 2022 |
| Base year | 2023 |
| Forecast period | 2024 - 2030 |
| Quantitative units | Revenue in USD million |
| Market segmentation | Product, Service |
| Key market players worldwide | AstraZeneca PLC, Caribou Biosciences Inc Ordinary Shares, Cellectis SA, CRISPR Therapeutics AG, Editas Medicine Inc, Roche Holding AG, PerkinElmer, Genscript Biotech Corp Class H, Danaher Corp, Intellia Therapeutics Inc, Lonza Group Ltd, Merck KGaA, Takara Bio Inc, Thermo Fisher Scientific Inc, Cibus Inc Ordinary Shares - Class A, Beam Therapeutics Inc, Vertex Pharmaceuticals Inc, Addgene, EGenesis, Synthego, Mammoth Biosciences, Inscripta, PLANTEDIT, Hera Biotech, Origene Technologies, Recombinetics |
Other key industry trends
- In terms of revenue, Mexico accounted for 0.3% of the global crispr and cas genes market in 2023.
- Country-wise, U.S. is expected to lead the global market in terms of revenue in 2030.
- In Latin America, Brazil crispr and cas genes market is projected to lead the regional market in terms of revenue in 2030.
- Mexico is the fastest growing regional market in Latin America and is projected to reach USD 30.1 million by 2030.
Product was the largest segment with a revenue share of 78.3% in 2024. Horizon Databook has segmented the Mexico crispr and cas genes market based on product, service covering the revenue growth of each sub-segment from 2018 to 2030.
In Mexico, human genome editing and human genetic engineering fields are not explicitly regulated. The birth of the first three-parent baby, in September 2016, by using maternal spindle transfer for mitochondrial-replacement therapy raised several ethical concerns in this region.
Therefore, in Mexico, the clinical application of CRISPR genome editing technology is highly regulated and supervised. Moreover, penalties are imposed for use of procedures that have not been safety-tested in humans. In addition, there is no determined regulatory status of gene editing in plant products or plants in Mexico.
Gene-edited products are regulated under restrictive laws developed for transgenic GMOs. These factors limit the use of CRISPR in Mexico to some extent. However, several research laboratories have established a set of guidelines based on the international regulatory framework of CRISPR applications in clinical research studies, which is expected to positively influence ongoing research studies in Mexico.
No credit card required*
Horizon in a snapshot
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more
CRISPR And Cas Genes Market Scope
CRISPR And Cas Genes Market Companies
| Name | Profile | # Employees | HQ | Website |
|---|
Mexico CRISPR And Cas Genes Market Outlook Share, 2024 & 2030 (US$M)
Related regional statistics
Sign up - it's easy, and free!
Sign up and get instant basic access to databook, upgrade
when ready, or enjoy our
free plan indefinitely.
Included in Horizon account
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more

