Argentina Cell And Gene Therapy Clinical Trials Market Size & Outlook
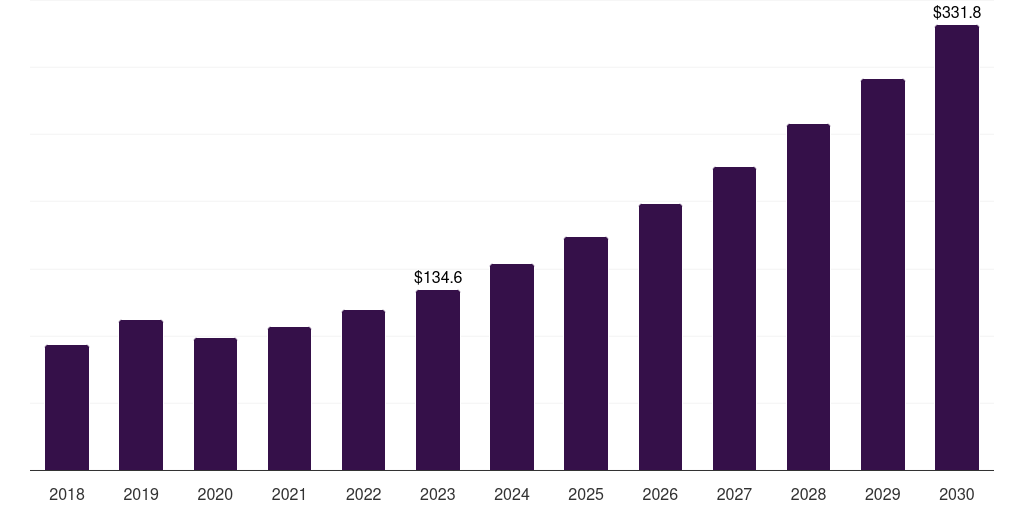
Argentina cell and gene therapy clinical trials market, 2018-2030 (US$M)
Related Markets
Argentina cell and gene therapy clinical trials market highlights
- The Argentina cell and gene therapy clinical trials market generated a revenue of USD 134.6 million in 2023 and is expected to reach USD 331.9 million by 2030.
- The Argentina market is expected to grow at a CAGR of 13.8% from 2024 to 2030.
- In terms of segment, phase ii was the largest revenue generating phase in 2023.
- Phase IV is the most lucrative phase segment registering the fastest growth during the forecast period.
Cell and gene therapy clinical trials market data book summary
| Market revenue in 2023 | USD 134.6 million |
| Market revenue in 2030 | USD 331.9 million |
| Growth rate | 13.8% (CAGR from 2023 to 2030) |
| Largest segment | Phase ii |
| Fastest growing segment | Phase IV |
| Historical data | 2018 - 2022 |
| Base year | 2023 |
| Forecast period | 2024 - 2030 |
| Quantitative units | Revenue in USD million |
| Market segmentation | Phase I, Phase II, Phase III, Phase IV |
| Key market players worldwide | IQVIA Holdings Inc, Icon PLC, Labcorp Holdings Inc, Charles River Laboratories International Inc, PAREXEL, Syneos Health, Medpace Holdings Inc, Thermo Fisher Scientific Inc, Veristat, Novotech |
Other key industry trends
- In terms of revenue, Argentina accounted for 1.4% of the global cell and gene therapy clinical trials market in 2023.
- Country-wise, U.S. is expected to lead the global market in terms of revenue in 2030.
- In Latin America, Brazil cell and gene therapy clinical trials market is projected to lead the regional market in terms of revenue in 2030.
- Brazil is the fastest growing regional market in Latin America and is projected to reach USD 643.8 million by 2030.
No credit card required*
Horizon in a snapshot
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more
Cell And Gene Therapy Clinical Trials Market Scope
Cell And Gene Therapy Clinical Trials Market Companies
| Name | Profile | # Employees | HQ | Website |
|---|
Argentina cell and gene therapy clinical trials market outlook
The databook is designed to serve as a comprehensive guide to navigating this sector. The databook focuses on market statistics denoted in the form of revenue and y-o-y growth and CAGR across the globe and regions. A detailed competitive and opportunity analyses related to cell and gene therapy clinical trials market will help companies and investors design strategic landscapes.
Phase ii was the largest segment with a revenue share of 49.26% in 2023. Horizon Databook has segmented the Argentina cell and gene therapy clinical trials market based on phase i, phase ii, phase iii, phase iv covering the revenue growth of each sub-segment from 2018 to 2030.
Argentina was not well known as an outsourcing location until just a few years ago, but the combination of good healthcare infrastructure mainly national clinical trial standards compatible with international norms as well as easy recruitment of patients has led to speedy growth in the CRO industry in Argentina. The government has also set up a series of sites of government-run clinical investigative sites (ANMAT sites) in high population urban areas.
The biotechnology and pharmaceutical industries are also preferring Argentina for clinical research due to easy patient recruitment. Also, people in Argentina do not have health coverage and in some cases has very limited access to basic healthcare. Hence, there are huge treatment-naïve potential patients to recruit for cell and gene therapy clinical trials.
Also, a large number of CROs such as Charles Liver Laboratories; Latin Clin; EGCP; Quintiles: Blanchard & Asociados; and Active have operations in Argentina. The Ministry of Health in Argentina issued a new regulation that reduces the timeline for the approval of cell and gene therapy clinical trials from 16 working days to 70 days or less.
Reasons to subscribe to Argentina cell and gene therapy clinical trials market databook:
-
Access to comprehensive data: Horizon Databook provides over 1 million market statistics and 20,000+ reports, offering extensive coverage across various industries and regions.
-
Informed decision making: Subscribers gain insights into market trends, customer preferences, and competitor strategies, empowering informed business decisions.
-
Cost-Effective solution: It's recognized as the world's most cost-effective market research database, offering high ROI through its vast repository of data and reports.
-
Customizable reports: Tailored reports and analytics allow companies to drill down into specific markets, demographics, or product segments, adapting to unique business needs.
-
Strategic advantage: By staying updated with the latest market intelligence, companies can stay ahead of competitors, anticipate industry shifts, and capitalize on emerging opportunities.
Target buyers of Argentina cell and gene therapy clinical trials market databook
-
Our clientele includes a mix of cell and gene therapy clinical trials market companies, investment firms, advisory firms & academic institutions.
-
30% of our revenue is generated working with investment firms and helping them identify viable opportunity areas.
-
Approximately 65% of our revenue is generated working with competitive intelligence & market intelligence teams of market participants (manufacturers, service providers, etc.).
-
The rest of the revenue is generated working with academic and research not-for-profit institutes. We do our bit of pro-bono by working with these institutions at subsidized rates.
Horizon Databook provides a detailed overview of country-level data and insights on the Argentina cell and gene therapy clinical trials market , including forecasts for subscribers. This country databook contains high-level insights into Argentina cell and gene therapy clinical trials market from 2018 to 2030, including revenue numbers, major trends, and company profiles.
Partial client list
Argentina cell and gene therapy clinical trials market size, by phase, 2018-2030 (US$M)
Argentina Cell And Gene Therapy Clinical Trials Market Outlook Share, 2023 & 2030 (US$M)
Related industry reports
Related regional statistics
Sign up - it's easy, and free!
Sign up and get instant basic access to databook, upgrade
when ready, or enjoy our
free plan indefinitely.
Included in Horizon account
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more



