U.S. Antifungal Drugs Market Size & Outlook, 2023-2030
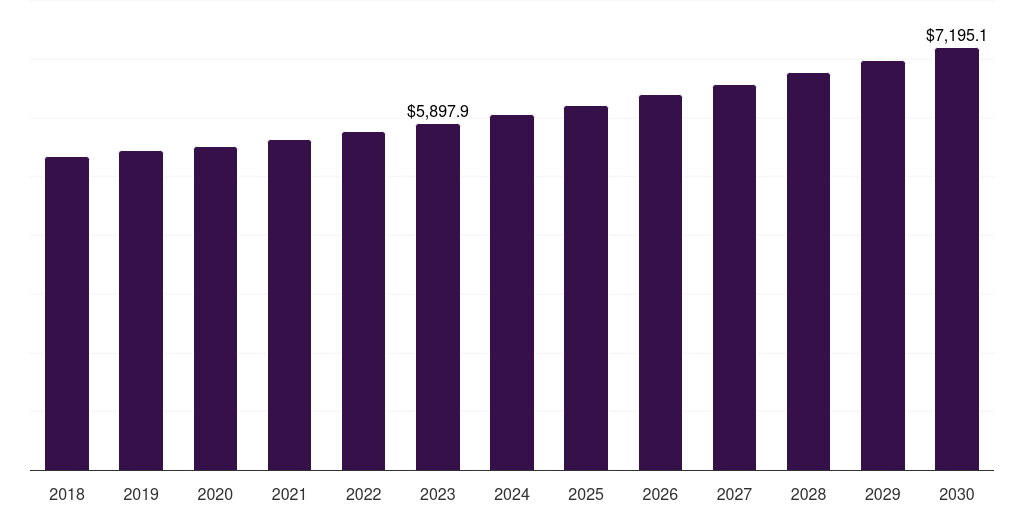
U.S. antifungal drugs market, 2018-2030 (US$M)
Related Markets
U.S. antifungal drugs market highlights
- The U.S. antifungal drugs market generated a revenue of USD 5,897.9 million in 2023 and is expected to reach USD 7,195.1 million by 2030.
- The U.S. market is expected to grow at a CAGR of 2.9% from 2024 to 2030.
- In terms of segment, azoles was the largest revenue generating drug class in 2023.
- Echinocandins is the most lucrative drug class segment registering the fastest growth during the forecast period.
Antifungal drugs market data book summary
| Market revenue in 2023 | USD 5,897.9 million |
| Market revenue in 2030 | USD 7,195.1 million |
| Growth rate | 2.9% (CAGR from 2023 to 2030) |
| Largest segment | Azoles |
| Fastest growing segment | Echinocandins |
| Historical data | 2018 - 2022 |
| Base year | 2023 |
| Forecast period | 2024 - 2030 |
| Quantitative units | Revenue in USD million |
| Market segmentation | Azoles, Echinocandins, Polyenes, Allylamines |
| Key market players worldwide | Novartis AG ADR, Pfizer Inc, Bayer AG, Sanofi SA, Merck KGaA, Merck & Co Inc, GlaxoSmithKline, Abbott Laboratories, Enzon Pharmaceuticals Inc, Astellas Pharma Inc, Glenmark Pharmaceuticals |
Other key industry trends
- In terms of revenue, U.S. accounted for 37.3% of the global antifungal drugs market in 2023.
- Country-wise, U.S. is expected to lead the global market in terms of revenue in 2030.
- In North America, U.S. antifungal drugs market is projected to lead the regional market in terms of revenue in 2030.
- Canada is the fastest growing regional market in North America and is projected to reach USD 755.3 million by 2030.
Azoles was the largest segment with a revenue share of 47.31% in 2023. Horizon Databook has segmented the U.S. antifungal drugs market based on azoles, echinocandins, polyenes, allylamines covering the revenue growth of each sub-segment from 2018 to 2030.
The FDA governs the approval for the marketing of antifungals and new drugs for human use. The antibiotics and antifungals are approved under New Drug and Antibiotic Regulations, 1985. FDA's Center for Drug Evaluation and Research (CDER) evaluates new drugs before they can enter the market.
The drug has to undergo laboratory and animal tests before entering human trials. The proven benefits of the drug must be more than its potential risks. A company seeking drug approval for a prescription pharmaceutical typically undergoes a five-step process, which includes drug discovery & concept, preclinical research phase, clinical research or clinical trials, FDA review process, and postmarketing monitoring of safety.
Accelerated approvals are also given by FDA depending on the unmet medical needs and data. For generic drugs, an Abbreviated New Drug Application (ANDA) has to be filed after the expiry of the patent. They do not have to go through the phases of clinical trials again, however, the company has to prove the bioequivalence of the product.
No credit card required*
Horizon in a snapshot
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more
Antifungal Drugs Market Scope
Antifungal Drugs Market Companies
| Name | Profile | # Employees | HQ | Website |
|---|
U.S. antifungal drugs market size, by drug class, 2018-2030 (US$M)
U.S. Antifungal Drugs Market Outlook Share, 2023 & 2030 (US$M)
Related regional statistics
No records
No related regions found.
Sign up - it's easy, and free!
Sign up and get instant basic access to databook, upgrade
when ready, or enjoy our
free plan indefinitely.
Included in Horizon account
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more


