North America Women's Health Market Size & Outlook
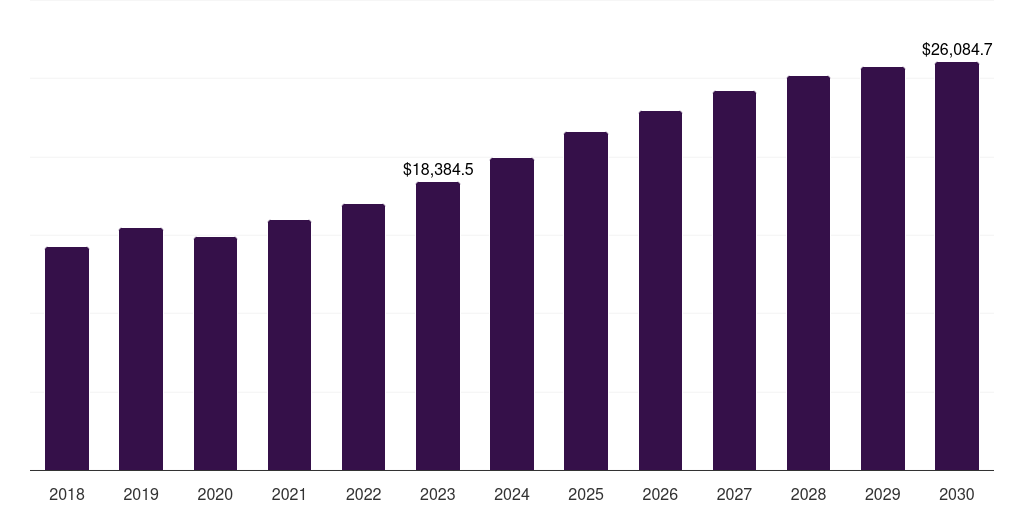
North America women's health market, 2018-2030 (US$M)
Related Markets

Increase in the population of older women in the region is one of the key factors driving the demand for women’s health products in North America. More than 15% of the women in North America are above the age of 65 years.
Moreover, increasing prevalence of osteoporosis in postmenopausal women is a key factor expected to drive the demand for women’s health products, including drugs and devices. Increase in the number of market approvals of women’s health drugs and high healthcare expenditure are among the factors contributing to market growth.
For instance, in May 2023, the U.S. FDA approved Veozah (fezolinetant) to reduce the frequency and severity of hot flashes in menopause. Moreover, in May 2020, AbbVie’s Oriahnn received FDA approval. It is an oral medication indicated for heavy menstrual bleeding due to uterine fibroids.
No credit card required*
Horizon in a snapshot
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more
Women's Health Market Scope
Women's Health Market Companies
| Name | Profile | # Employees | HQ | Website |
|---|
North America Women's Health Market Outlook Share, 2024 & 2030 (US$M)
Related industry reports
Related regional statistics
Sign up - it's easy, and free!
Sign up and get instant basic access to databook, upgrade
when ready, or enjoy our
free plan indefinitely.
Included in Horizon account
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more

