South Korea Recombinant Protein Therapeutics Cdmo Market Size & Outlook
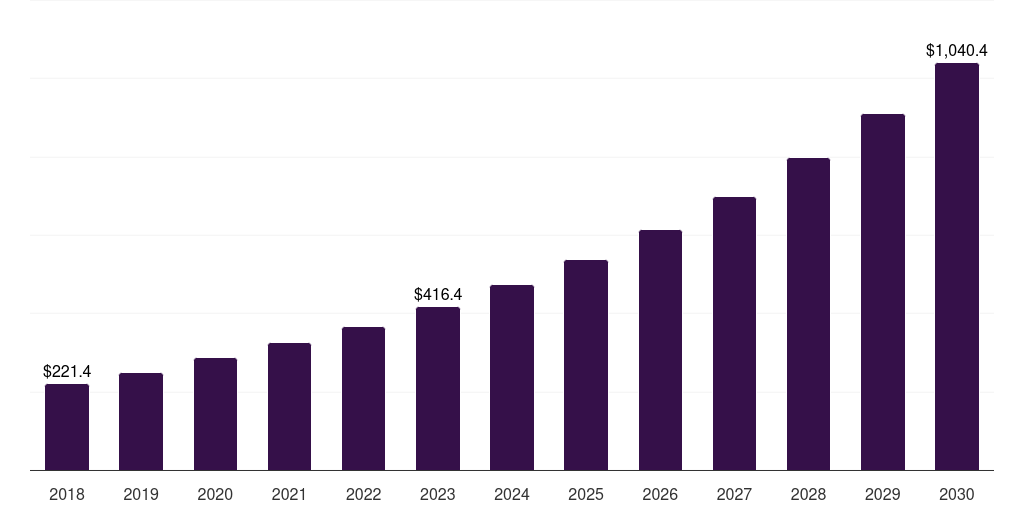
South Korea recombinant protein therapeutics cdmo market, 2018-2030 (US$M)
Related Markets
South Korea recombinant protein therapeutics cdmo market highlights
- The South Korea recombinant protein therapeutics cdmo market generated a revenue of USD 416.4 million in 2023 and is expected to reach USD 1,040.4 million by 2030.
- The South Korea market is expected to grow at a CAGR of 14% from 2024 to 2030.
- In terms of segment, interferons was the largest revenue generating type in 2023.
- Growth Hormones is the most lucrative type segment registering the fastest growth during the forecast period.
Recombinant protein therapeutics cdmo market data book summary
| Market revenue in 2023 | USD 416.4 million |
| Market revenue in 2030 | USD 1,040.4 million |
| Growth rate | 14% (CAGR from 2023 to 2030) |
| Largest segment | Interferons |
| Fastest growing segment | Growth Hormones |
| Historical data | 2018 - 2022 |
| Base year | 2023 |
| Forecast period | 2024 - 2030 |
| Quantitative units | Revenue in USD million |
| Market segmentation | Growth Hormones, Interferons, Vaccines, Immunostimulating Agents |
| Key market players worldwide | Lonza Group Ltd, Catalent Inc, FUJIFILM Holdings Corp, WuXi Biologics (Cayman) Inc, Batavia Biosciences, Richter-Helm, Curia, HALIX, Biovian, Enzene Biosciences |
Other key industry trends
- In terms of revenue, South Korea accounted for 2.0% of the global recombinant protein therapeutics cdmo market in 2023.
- Country-wise, U.S. is expected to lead the global market in terms of revenue in 2030.
- In Asia Pacific, Japan recombinant protein therapeutics cdmo market is projected to lead the regional market in terms of revenue in 2030.
- China is the fastest growing regional market in Asia Pacific and is projected to reach USD 2,968.6 million by 2030.
Interferons was the largest segment with a revenue share of 21.4% in 2023. Horizon Databook has segmented the South Korea recombinant protein therapeutics cdmo market based on growth hormones, interferons, vaccines, immunostimulating agents covering the revenue growth of each sub-segment from 2018 to 2030.
The increasing government support is expected to improve opportunities for drug development companies. In recent years, South Korea has emerged as a notable force in science funding. Substantial government expenditures and strong private investments have propelled total R&D support from approximately 3.9% of GDP a decade ago to over 4.9% in 2022.
In addition, the government’s support for developing a robust clinical trial environment is expected to boost the demand for advanced therapeutics, contributing to market growth. The recombinant protein therapeutics CDMO market in South Korea is expected to exhibit lucrative growth in the coming years, which can be attributed to the growing demand for advanced therapies and rapid advancements in the CDMO industry in the past few decades.
Moreover, the country is a prominent destination for clinical trials in Asia Pacific, with its superior medical infrastructure and a highly educated, relatively healthy & wealthy population. In addition, the increasing government support has led to a rise in opportunities for recombinant protein therapeutics CDMOs. For instance, the South Korean government has formed a public–private consultative body that conducts bioequivalence tests for generic drugs. This is expected to improve the quality of generic drugs in South Korea and, thus, boost demand for CDMOs.
No credit card required*
Horizon in a snapshot
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more
Recombinant Protein Therapeutics CDMO Market Scope
Recombinant Protein Therapeutics CDMO Market Companies
| Name | Profile | # Employees | HQ | Website |
|---|
South Korea recombinant protein therapeutics cdmo market size, by type, 2018-2030 (US$M)
South Korea Recombinant Protein Therapeutics CDMO Market Outlook Share, 2023 & 2030 (US$M)
Related industry reports
Related regional statistics
Sign up - it's easy, and free!
Sign up and get instant basic access to databook, upgrade
when ready, or enjoy our
free plan indefinitely.
Included in Horizon account
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more


