Canada Pharmacovigilance Market Size & Outlook, 2023-2030
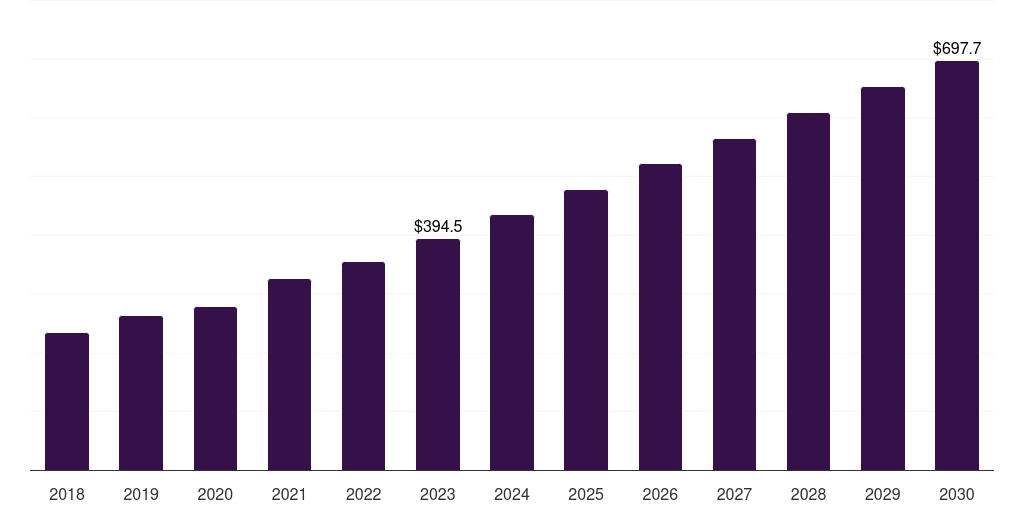
Canada pharmacovigilance market, 2018-2030 (US$M)
Related Markets
Canada pharmacovigilance market highlights
- The Canada pharmacovigilance market generated a revenue of USD 394.5 million in 2023 and is expected to reach USD 697.7 million by 2030.
- The Canada market is expected to grow at a CAGR of 8.5% from 2024 to 2030.
- In terms of segment, phase 4 was the largest revenue generating product life cycle in 2023.
- Phase 3 is the most lucrative product life cycle segment registering the fastest growth during the forecast period.
Pharmacovigilance market data book summary
| Market revenue in 2023 | USD 394.5 million |
| Market revenue in 2030 | USD 697.7 million |
| Growth rate | 8.5% (CAGR from 2023 to 2030) |
| Largest segment | Phase 4 |
| Fastest growing segment | Phase 3 |
| Historical data | 2018 - 2022 |
| Base year | 2023 |
| Forecast period | 2024 - 2030 |
| Quantitative units | Revenue in USD million |
| Market segmentation | Pre Clinical, Phase 1, Phase 2, Phase 3, Phase 4 |
| Key market players worldwide | Accenture PLC Class A, IQVIA Holdings Inc, Cognizant Technology Solutions Corp Class A, Linical Americas, International Business Machines Corp, Labcorp Holdings Inc, ArisGlobal, Capgemini SE, ITClinical, Icon PLC, TAKE Solutions, PAREXEL, Wipro Ltd ADR, United Biosource Corporation, FMD K&L, BioClinica |
Other key industry trends
- In terms of revenue, Canada accounted for 5.4% of the global pharmacovigilance market in 2023.
- Country-wise, U.S. is expected to lead the global market in terms of revenue in 2030.
- In North America, U.S. pharmacovigilance market is projected to lead the regional market in terms of revenue in 2030.
- Canada is the fastest growing regional market in North America and is projected to reach USD 697.7 million by 2030.
Phase 4 was the largest segment with a revenue share of 75.97% in 2023. Horizon Databook has segmented the Canada pharmacovigilance market based on pre clinical, phase 1, phase 2, phase 3, phase 4 covering the revenue growth of each sub-segment from 2018 to 2030.
Canada is a small market for PV owing to its small population size. The Canadian government started Health Canada long back in 1996 to improve the healthcare infrastructure in the country. Health Canada authorizes over 900 clinical trials every year. The organization maintains a clinical trial database that provides information related to clinical trials related to human pharmaceuticals and biologics.
Moreover, Health Canada has started an online platformed Effect Canada and Canada Vigilance Program—for reporting any ADR. Canadian Clinical Trials Coordinating Centre (CCTCC), a collaborative organization between the government and the Canadian pharmaceutical industry, was created to strengthen the clinical trials environment and promote Canada as a leading destination for clinical trials, which is also aiding market growth.
No credit card required*
Horizon in a snapshot
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more
Pharmacovigilance Market Scope
Pharmacovigilance Market Companies
| Name | Profile | # Employees | HQ | Website |
|---|
Canada pharmacovigilance market size, by product life cycle, 2018-2030 (US$M)
Canada Pharmacovigilance Market Outlook Share, 2023 & 2030 (US$M)
Related industry reports
Related regional statistics
Sign up - it's easy, and free!
Sign up and get instant basic access to databook, upgrade
when ready, or enjoy our
free plan indefinitely.
Included in Horizon account
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more


