North America Medical Device Validation & Verification Market Size & Outlook
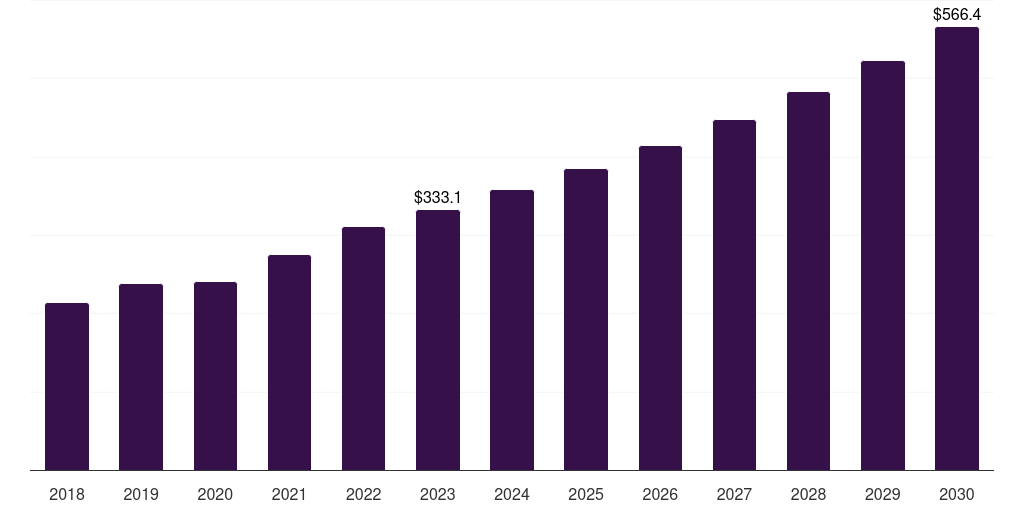
North America medical device validation & verification market, 2018-2030 (US$M)
Related Markets
North America medical device validation & verification market highlights
- The North America medical device validation & verification market generated a revenue of USD 333.1 million in 2023.
- The market is expected to grow at a CAGR of 7.9% from 2024 to 2030.
- In terms of segment, oncology was the largest revenue generating therapeutic area in 2023.
- Oncology is the most lucrative therapeutic area segment registering the fastest growth during the forecast period.
- Country-wise, U.S. is expected to register the highest CAGR from 2024 to 2030.
North America data book summary
| Market revenue in 2023 | USD 333.1 million |
| Market revenue in 2030 | USD 566.4 million |
| Growth rate | 7.9% (CAGR from 2023 to 2030) |
| Largest segment | Oncology |
| Fastest growing segment | Oncology |
| Historical data covered | 2018 - 2022 |
| Base year for estimation | 2023 |
| Forecast period covered | 2024 - 2030 |
| Quantitative units | Revenue in USD million |
| Market segmentation | Cardiovascular, Dermatology, Orthopedics, Nephrology, Respiratory, Neurology, Oncology, ENT |
| Key market players worldwide | SGS AG, Intertek Group PLC, Element Materials Technology, NAMSA, Eurofins Scientific SE, Charles River Laboratories International Inc, Sterling Check Corp, Toxikon, Steris PLC |
Other key industry trends
- In terms of revenue, North America region accounted for 45.2% of the global medical device validation & verification market in 2023.
- Globally, North America is projected to lead the regional market in terms of revenue in 2030.
- Asia Pacific is the fastest growing regional market and is projected to reach USD 226.6 million by 2030.
No credit card required*
Horizon in a snapshot
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more
Medical Device Validation & Verification Market Scope
Medical Device Validation & Verification Market Companies
| Name | Profile | # Employees | HQ | Website |
|---|
North America medical device validation & verification market outlook
The databook is designed to serve as a comprehensive guide to navigating this sector. The databook focuses on market statistics denoted in the form of revenue and y-o-y growth and CAGR across the globe and regions. A detailed competitive and opportunity analyses related to medical device validation & verification market will help companies and investors design strategic landscapes.
Oncology was the largest segment with a revenue share of 26.54% in 2023. Horizon Databook has segmented the North America medical device validation & verification market based on cardiovascular, dermatology, orthopedics, nephrology, respiratory, neurology, oncology, ent covering the revenue growth of each sub-segment from 2018 to 2030.
- North America Medical Device Validation & Verification Therapeutic Area Outlook (Revenue, USD Million, 2018-2030)
- Cardiovascular
- Dermatology
- Orthopedics
- Nephrology
- Respiratory
- Neurology
- Oncology
- ENT
- Others
- North America Medical Device Validation & Verification Application Outlook (Revenue, USD Million, 2018-2030)
- Diagnostics
- Therapeutic
- Implants
- Active Implantable Medical Device
- Medical Implants
- North America Medical Device Validation & Verification Technology Outlook (Revenue, USD Million, 2018-2030)
- Mechanical testing
- Biological
- EMI/EMC
- Electrical safety testing
Reasons to subscribe to North America medical device validation & verification market databook:
-
Access to comprehensive data: Horizon Databook provides over 1 million market statistics and 20,000+ reports, offering extensive coverage across various industries and regions.
-
Informed decision making: Subscribers gain insights into market trends, customer preferences, and competitor strategies, empowering informed business decisions.
-
Cost-Effective solution: It's recognized as the world's most cost-effective market research database, offering high ROI through its vast repository of data and reports.
-
Customizable reports: Tailored reports and analytics allow companies to drill down into specific markets, demographics, or product segments, adapting to unique business needs.
-
Strategic advantage: By staying updated with the latest market intelligence, companies can stay ahead of competitors, anticipate industry shifts, and capitalize on emerging opportunities.
Target buyers of North America medical device validation & verification market databook
-
Our clientele includes a mix of medical device validation & verification market companies, investment firms, advisory firms & academic institutions.
-
30% of our revenue is generated working with investment firms and helping them identify viable opportunity areas.
-
Approximately 65% of our revenue is generated working with competitive intelligence & market intelligence teams of market participants (manufacturers, service providers, etc.).
-
The rest of the revenue is generated working with academic and research not-for-profit institutes. We do our bit of pro-bono by working with these institutions at subsidized rates.
Horizon Databook provides a detailed overview of continent-level data and insights on the North America medical device validation & verification market , including forecasts for subscribers. This continent databook contains high-level insights into North America medical device validation & verification market from 2018 to 2030, including revenue numbers, major trends, and company profiles.
Partial client list
North America medical device validation & verification market size, by country, 2018-2030 (US$M)
North America Medical Device Validation & Verification Market Outlook Share, 2023 & 2030 (US$M)
Related industry reports
Related regional statistics
Sign up - it's easy, and free!
Sign up and get instant basic access to databook, upgrade
when ready, or enjoy our
free plan indefinitely.
Included in Horizon account
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more



