Thailand Cell Culture Media Market Size & Outlook
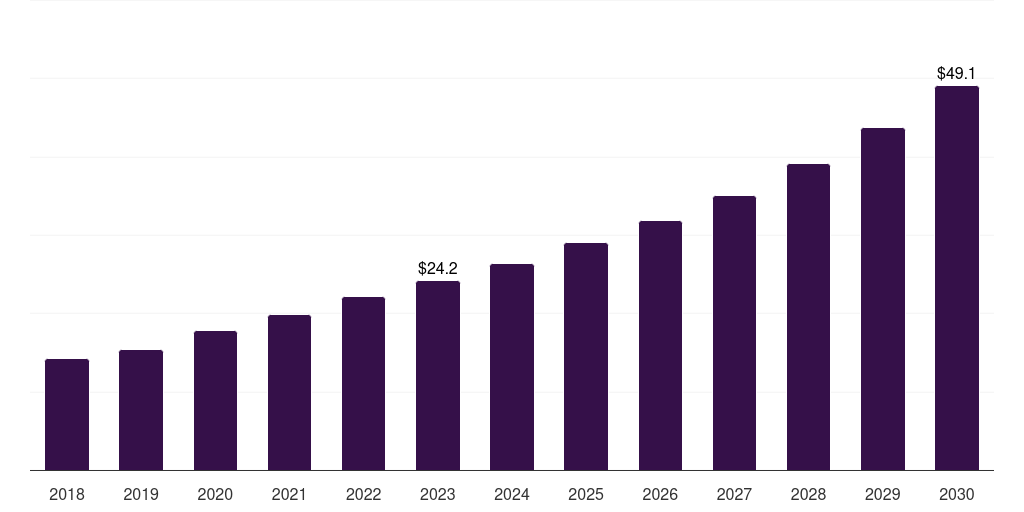
Thailand cell culture media market, 2018-2030 (US$M)
Related Markets
Thailand cell culture media market highlights
- The Thailand cell culture media market generated a revenue of USD 21.6 million in 2024 and is expected to reach USD 38.7 million by 2030.
- The Thailand market is expected to grow at a CAGR of 10.6% from 2025 to 2030.
- In terms of segment, serum-free media was the largest revenue generating product in 2024.
- Serum-free Media is the most lucrative product segment registering the fastest growth during the forecast period.
Cell culture media market data book summary
| Market revenue in 2024 | USD 21.6 million |
| Market revenue in 2030 | USD 38.7 million |
| Growth rate | 10.6% (CAGR from 2025 to 2030) |
| Largest segment | Serum-free media |
| Fastest growing segment | Serum-free Media |
| Historical data | 2018 - 2023 |
| Base year | 2024 |
| Forecast period | 2025 - 2030 |
| Quantitative units | Revenue in USD million |
| Market segmentation | Serum-free Media, Classical Media, Stem Cell Culture Media, Chemically Defined Media, Specialty Media, Other Cell Culture Media |
| Key market players worldwide | Sartorius AG, Danaher Corp, Merck KGaA, Thermo Fisher Scientific Inc, FUJIFILM Holdings Corp, Lonza Group Ltd, BD, STEMCELL Technologies, PromoCell GmbH |
Other key industry trends
- In terms of revenue, Thailand accounted for 0.5% of the global cell culture media market in 2024.
- Country-wise, U.S. is expected to lead the global market in terms of revenue in 2030.
- In Asia Pacific, China cell culture media market is projected to lead the regional market in terms of revenue in 2030.
- China is the fastest growing regional market in Asia Pacific and is projected to reach USD 663.4 million by 2030.
Serum-free media was the largest segment with a revenue share of 38.43% in 2024. Horizon Databook has segmented the Thailand cell culture media market based on serum-free media, classical media, stem cell culture media, chemically defined media, specialty media, other cell culture media covering the revenue growth of each sub-segment from 2018 to 2030.
Medicinal drugs in Thailand are governed by the Ministry of Public Health (MOPH). The MOPH consists of the Drug Control Division of the Food and Drug Administration (FDA) department, which manages the four main aspects of drug regulation, including pre-marketing control (licensing, registration, and other processes), post-marketing monitoring & surveillance, national standards & consumer education, and promotion of development & research.
The country also follows separate licensing procedures for herbal/traditional medicines and modern medicines. Although a centralized regulation for controlling clinical trials is not present in Thailand, the Drug Act No. 6 B.E. 2562 (2019) enables indirect supervision of the Thai FDA for controlling clinical studies in which the investigational products are imported in Thailand.
Furthermore, at least six regulatory authorities, including the FDA of the MOPH, Ethical Review Committee for Research in Human Subjects of the MOPH, Department of Medical Sciences of the MOPH, Department of Communicable Diseases Control of the MOPH, National Sub-Committee of HIV Vaccine of the MOPH, and medical schools & hospitals with specific regulations have jurisdiction over various aspects of clinical trials.
No credit card required*
Horizon in a snapshot
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more
Cell Culture Media Market Scope
Cell Culture Media Market Companies
| Name | Profile | # Employees | HQ | Website |
|---|
Thailand cell culture media market size, by product, 2018-2030 (US$M)
Thailand Cell Culture Media Market Outlook Share, 2024 & 2030 (US$M)
Related regional statistics
Sign up - it's easy, and free!
Sign up and get instant basic access to databook, upgrade
when ready, or enjoy our
free plan indefinitely.
Included in Horizon account
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more


