Canada Cell Culture Media Market Size & Outlook, 2024-2030
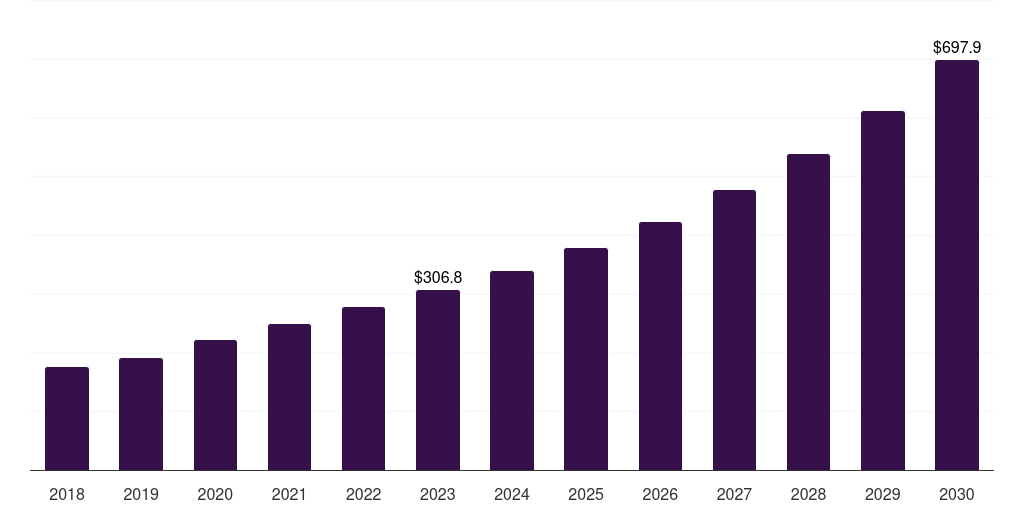
Canada cell culture media market, 2018-2030 (US$M)
Related Markets
Canada cell culture media market highlights
- The Canada cell culture media market generated a revenue of USD 277.4 million in 2024 and is expected to reach USD 550.2 million by 2030.
- The Canada market is expected to grow at a CAGR of 12.5% from 2025 to 2030.
- In terms of segment, serum-free media was the largest revenue generating product in 2024.
- Serum-free Media is the most lucrative product segment registering the fastest growth during the forecast period.
Cell culture media market data book summary
| Market revenue in 2024 | USD 277.4 million |
| Market revenue in 2030 | USD 550.2 million |
| Growth rate | 12.5% (CAGR from 2025 to 2030) |
| Largest segment | Serum-free media |
| Fastest growing segment | Serum-free Media |
| Historical data | 2018 - 2023 |
| Base year | 2024 |
| Forecast period | 2025 - 2030 |
| Quantitative units | Revenue in USD million |
| Market segmentation | Serum-free Media, Classical Media, Stem Cell Culture Media, Chemically Defined Media, Specialty Media, Other Cell Culture Media |
| Key market players worldwide | Sartorius AG, Danaher Corp, Merck KGaA, Thermo Fisher Scientific Inc, FUJIFILM Holdings Corp, Lonza Group Ltd, BD, STEMCELL Technologies, PromoCell GmbH |
Other key industry trends
- In terms of revenue, Canada accounted for 6.4% of the global cell culture media market in 2024.
- Country-wise, U.S. is expected to lead the global market in terms of revenue in 2030.
- In North America, U.S. cell culture media market is projected to lead the regional market in terms of revenue in 2030.
- Canada is the fastest growing regional market in North America and is projected to reach USD 550.2 million by 2030.
Serum-free media was the largest segment with a revenue share of 42.43% in 2024. Horizon Databook has segmented the Canada cell culture media market based on serum-free media, classical media, stem cell culture media, chemically defined media, specialty media, other cell culture media covering the revenue growth of each sub-segment from 2018 to 2030.
Canada's regulatory approach involves three sets of regulations, namely the Cells, Tissues and Organs Regulations; the Food and Drug Regulations; and the Medical Devices Regulations. In Canada, CGT products are regulated under the existing regulatory frameworks developed for pharmaceutical & biologic drugs, human material for transplants, and medical devices.
A majority of CGTs are classified as biologics and are regulated by the Biologic and Radiopharmaceutical Drugs Directorate (BRDD) within Health Canada. As a biological drug, the sponsor of a CGT must submit a Clinical Trial Application (CTA) to Health Canada prior to commencing a clinical trial, and file a New Drug Submission (NDS) for review and approval prior to marketing a new therapy in Canada.
No credit card required*
Horizon in a snapshot
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more
Cell Culture Media Market Scope
Cell Culture Media Market Companies
| Name | Profile | # Employees | HQ | Website |
|---|
Canada cell culture media market size, by product, 2018-2030 (US$M)
Canada Cell Culture Media Market Outlook Share, 2024 & 2030 (US$M)
Related regional statistics
Sign up - it's easy, and free!
Sign up and get instant basic access to databook, upgrade
when ready, or enjoy our
free plan indefinitely.
Included in Horizon account
- 30K+ Global Market Reports
- 120K+ Country Reports
- 1.2M+ Market Statistics
- 200K+ Company Profiles
- Industry insights and more


