- Home
- »
- Market Trend Reports
- »
-
Monoclonal Antibody Drugs and Indication
![Monoclonal Antibody Drugs and IndicationReport]()
Monoclonal Antibody Drugs and Indication
- Published: Jun, 2023
- Report ID: GVR-MT-100122
- Format: PDF, Horizon Databook
- Number of Pages: 0
- Report Coverage: 2024 - 2030
Overview
The field of Monoclonal Antibodies (MAbs) is experiencing rapid growth in the pharmaceutical industry, making it one of the most dynamic areas of new drug development. Over the next decade, we can expect a significant increase in the utilization and adoption of monoclonal antibody products. In recent years, several blockbuster products such as Rituxan, Remicade, Avastin, Humira, and Herceptin, have received approval and achieved blockbuster status. Furthermore, there are currently over 300 new drugs in clinical trials, indicating the ongoing innovation in this field. Both established biotechnology companies and emerging start-ups are actively involved in the development of monoclonal antibodies. This collaborative effort will contribute to the growing prominence of monoclonal antibody products in the coming years.
MAbs are extremely selective for cancer cells because they attach to proteins on their surfaces & stimulate an immune reaction. Cancer mAbs may hold a significant position within the global mAbs market due to the growing global prevalence of cancer. Moreover, major pharmaceutical companies worldwide are making substantial investments in research and development related to cancer biologics, particularly MAbs. This is because MAbs offer efficiency and reduced toxicity compared to chemotherapy and other treatment modalities for various types of cancer, making them valuable in both the diagnosis and treatment of various cancer types.
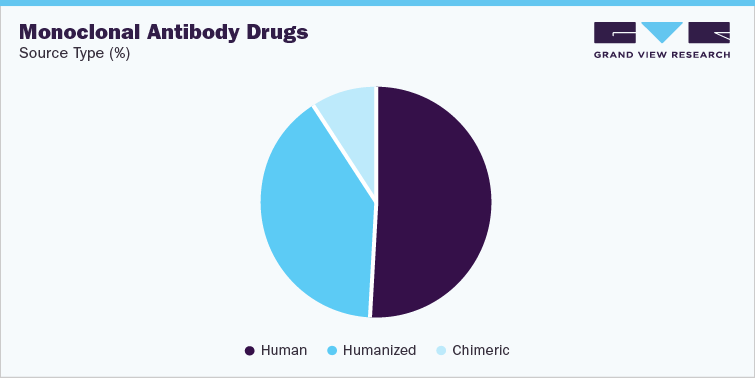
Human mAbs are preferred due to lower immunogenicity as compared to other mAb types such as murine, chimeric, and others. The first fully human mAb, Humira, was approved in 2002 with the help of phage display technology and the number of approved fully human mAbs has continued to rise ever since. A majority of the fully human mAbs are produced using transgenic mice, while some are produced by using phage display technology. Rising adoption of transgenic mice is favored by their ability to produce highly specific and affinity-matured antibodies, whereas demand for phage display technologies is supported by scalability, parallelization, and miniaturization attributes offered by the technique. Furthermore, fully human mAbs represent a promising option for treatment of severe, nonmalignant conditions, such as asthma, hypercholesterolemia, atopic dermatitis, and osteoporosis, which can significantly boost market growth. For instance, in December 2022, Aptar Pharma stated that its VP7 nasal multi-dose system will be used to deliver Hibiocy's Vaill Covitrap anti-CoV decongestant spray, which has been recently licensed by Thailand's Food & Drug Administration (FDA).
Topics Covered Under This Study
Attribute
Details
List of top Monoclonal Antibodies
- Generic Name
- Company
- Source type
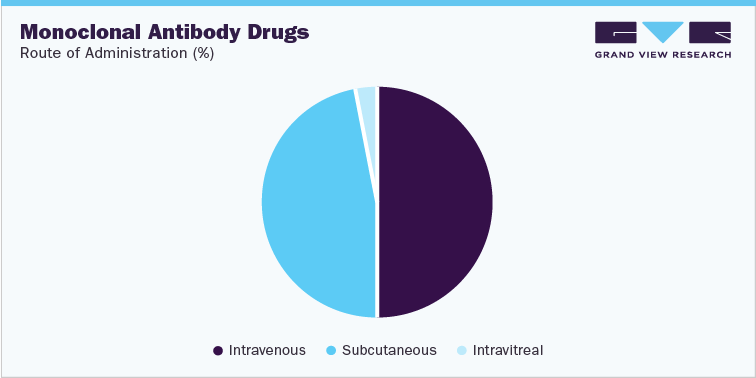
- Route of Administration
- Indication
Financial Performance
- Product wise Revenue
Heat Map Analysis, By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Top Monoclonal Antibodies, 2022
Table 1 List of top monoclonal Antibodies
Sr. No.
Product
Generic Name
Company
Source Type
Route of Administration
Indication
1
Humira
Adalimumab
AbbVie
Human
Subcutaneous
Rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, plaque psoriasis, hidradenitis suppurativa, uveitis, and juvenile idiopathic arthritis
2
Skyrizi
Risankizumab
AbbVie, Boehringer Ingelheim
Humanized
Subcutaneous
Plaque psoriasis, Crohn's disease
3
Herceptin
Trastuzumab
Roche
Humanized
Intravenous, subcutaneous
Breast cancer, Gastric cancer
4
Avastin
Bevacizumab
Roche
Humanized
Intravenous
Non-small cell lung cancer, Breast ERB2 negative cancer, Renal cell carcinoma, Giloblastoma
5
Mab Thera/Rituxan
Rituximab
Roche
Chimeric
Intravenous
Non-Hodgkin’s Lymphoma, B-cell acute leukemia, Chronic Lymphocytic Leukemia (CLL), Rheumatoid arthritis (RA), Granulomatosis with Polyangiitis (GPA), Pemphigus Vulgaris (PV)
6
Perjeta
Pertuzumab
Roche
Humanized
Intravenous
Breast cancer
7
Ocrevus
Ocrelizumab
Roche
Humanized
Intravenous
Multiple sclerosis
8
Tecentriq
Atezolizumab
Roche
Humanized
Intravenous
Urothelial carcinoma, non-small cell lung cancer, triple-negative breast cancer, small cell lung cancer, hepatocellular carcinoma, and alveolar soft part sarcoma.
9
Hemlibra
Emicizumab
Roche
Humanized
Subcutaneous
Haemophilia A
10
Gazyvaro
Obinutuzumab
Roche
Humanized
Intravenous
Chronic lymphocytic leukemia (CLL)
11
Xolair
Omalizumab
Roche
Humanized
Subcutaneous
Nasal polyps, allergic asthma & chronic spontaneous urticaria (CSU)
12
Lucentis
Ranibizumab
Roche
Humanized
Intravitreal
Age-related macular degeneration (AMD), diabetic eye disease
13
Actemra
Tocilizumab
Roche
Humanized
Intravenous
Rheumatoid arthritis (RA), giant cell arteritis (GCA), polyarticular juvenile idiopathic arthritis (PJIA), systemic juvenile idiopathic arthritis (SJIA), systemic sclerosis-associated interstitial lung disease (SSc-ILD)
14
Keytruda
Pembrolizumab
Merck & Co.
Human
Intravenous
Melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, cervical cancer, and certain types of breast cancer
15
Simponi
Golimumab
Merck & Co.
Human
Subcutaneous
Rheumatoid arthritis (RA), psoriatic arthritis, Active ankylosing spondylitis (AS), ulcerative colitis (UC),
16
Remicade
Infliximab
Merck & Co.
Chimeric
Intravenous
Rheumatoid arthritis, Ankylosing spondylitis, Ulcerative colitis, Psoriatic arthritis, Psoriasis
17
Remicade
Infliximab
Janssen Pharmaceuticals
Chimeric
Intravenous
Rheumatoid arthritis, Ankylosing spondylitis, Ulcerative colitis, Psoriatic arthritis, Psoriasis
18
Stelara
Ustekinumab
Janssen Pharmaceuticals
Human
Intravenous
Crohn's disease, ulcerative colitis, plaque psoriasis and psoriatic arthritis
19
Tremfya
Guselkumab
Janssen Pharmaceuticals
Human
Subcutaneous
Plaque psoriasis
20
Simponi
Golimumab
Janssen Pharmaceuticals
Human
Subcutaneous
Rheumatoid arthritis (RA), psoriatic arthritis, Active ankylosing spondylitis (AS), ulcerative colitis (UC),
21
Darzalex
Daratumumab
Janssen Pharmaceuticals
Human
Intravenous
Multiple myeloma, light chain (AL) amyloidosis
22
Opdivo
Nivolumab
Bristol-Meyers Squibb
Human
Intravenous
Esophageal cancer, gastroesophageal junction cancer, stomach cancer, colorectal cancer, non-small cell lung cancer, renal cell carcinoma (a type of kidney cancer), melanoma, hepatocellular carcinoma
23
Prolia
Denosumab
Amgen, Inc.
Human
Subcutaneous
Osteoporosis, treatment-induced bone loss, metastases to bone, and giant cell tumor of bone
24
XGEVA
Denosumab
Amgen, Inc.
Human
Subcutaneous
Osteoporosis, treatment-induced bone loss, metastases to bone, and giant cell tumor of bone
25
Repatha
Evolocumab
Amgen, Inc.
Human
Subcutaneous
Hyperlipidemia
26
Vectibix
Panitumumab
Amgen, Inc.
Human
Intravenous
Epidermal growth factor receptor (EGFR)-expressing, metastatic colorectal carcinoma (mCRC)
27
MVASI
Bevacizumab-awwb
Amgen, Inc.
Humanized
Intravenous
Metastatic colorectal cancer
28
KANJINTI
Trastuzumab-anns
Amgen, Inc.
Humanized
Intravenous
Early breast cancer
29
Aimovig
Erenumab
Amgen, Inc.
Human
Subcutaneous
Migraine
30
EVENITY
Romosozumab-aqqg
Amgen, Inc.
Humanized
Subcutaneous
Osteoporosis in women after menopause who are at high risk for fracture
31
Amgevita
Adalimumab
Amgen, Inc.
Human
Subcutaneous
Hidradenitis suppurativa (acne inversa), plaque psoriasis, psoriatic arthritis
32
Dupixent
Dupilumab
Regeneron
Human
Subcutaneous
Atopic Dermatitis, asthma, nasal polyps, eosinophilic esophagitis, and prurigo nodularis.
33
Libtayo
Cemiplimab
Regeneron
Human
Intravenous
Metastatic basal cell carcinoma, cutaneous squamous cell carcinoma (CSCC)
34
Kevzara
Sarilumab
Regeneron
Human
Subcutaneous
Active rheumatoid arthritis (RA)
35
Praluent
Alirocumab
Regeneron
Human
Subcutaneous
Cardiovascular disease to reduce the risk of heart attack, stroke, and certain types of chest pain conditions (unstable angina)
Top Monoclonal Antibodies: Financial Performance, 2018-2022 (USD Million)
Table 2 List of top monoclonal Antibodies
Sr. No.
Product
Company
Revenue (USD Million)
2018
2019
2020
2021
2022
1
Humira
AbbVie
19,900
19,200
19,800
21,000
21,237
2
Skyrizi
AbbVie, Boehringer Ingelheim
-
350
1,590
2,900
5,100
3
Herceptin
Roche
7,100
6,230
4,120
2,900
2,379
4
Avastin
Roche
6,950
7,310
5,570
3,400
2,356
5
Mab Thera/Rituxan
Roche
6,860
6,690
4,680
2,700
2,304
6
Perjeta
Roche
2,820
3,620
4,850
4,400
4,538
7
Ocrevus
Roche
2,390
3,830
6,110
5,500
6,703
8
Tecentriq
Roche
780
1,960
4,150
3,700
4,128
9
Hemlibra
Roche
220
1,450
3,670
3,300
4,245
10
Gazyvaro
Roche
400
570
700
800
900
11
Xolair
Roche
1,940
2,030
2,120
2,200
2,452
12
Lucentis
Roche
1,680
1,880
1,560
1,500
1,440
13
Actemra
Roche
2,200
2,380
3,230
4,200
2,999
14
Keytruda
Merck & Co.
7,200
11,100
14,400
17,400
20,937
15
Simponi
Merck & Co.
890
830
830
800
706
16
Remicade
Merck & Co.
580
410
330
300
207
17
Remicade
Janssen Pharmaceuticals
5,300
4,400
3,500
3,200
2,343
18
Stelara
Janssen Pharmaceuticals
5,200
6,400
7,700
9,300
9,723
19
Tremfya
Janssen Pharmaceuticals
500
1,000
1,300
2,000
2,668
20
Simponi
Janssen Pharmaceuticals
2,100
2,200
2,200
2,300
2,184
21
Darzalex
Janssen Pharmaceuticals
2,000
3,000
4,190
6,100
7,977
22
Opdivo
Bristol-Meyers Squibb
6,730
7,200
6,990
6,800
8,249
23
Prolia
Amgen, Inc.
2,300
2,700
2,800
3,200
3,628
24
XGEVA
Amgen, Inc.
1,800
1,900
1,900
2,000
2,014
25
Repatha
Amgen, Inc.
600
700
900
1,100
1,296
26
Vectibix
Amgen, Inc.
700
700
800
800
893
27
MVASI
Amgen, Inc.
-
100
800
1,100
900
28
KANJINTI
Amgen, Inc.
-
200
600
500
310
29
Aimovig
Amgen, Inc.
100
300
400
300
410
30
EVENITY
Amgen, Inc.
-
200
400
600
787
31
Amgevita
Amgen, Inc.
-
200
300
400
460
32
Dupixent
Regeneron
920
2,310
4,040
6,300
8,681
33
Libtayo
Regeneron
10
190
340
500
578
34
Kevzara
Regeneron
100
200
260
300
358
35
Praluent
Regeneron
300
280
350
400
467
Share this report with your colleague or friend.
GET A FREE SAMPLE
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
![gvr icn]()
NEED A CUSTOM REPORT?
We can customize every report - free of charge - including purchasing stand-alone sections or country-level reports, as well as offer affordable discounts for start-ups & universities.
Contact us now to get our best pricing.
![esomar icon]()
ESOMAR certified & member
![ISO]()
ISO Certified
We are GDPR and CCPA compliant! Your transaction & personal information is safe and secure. For more details, please read our privacy policy.
We are committed towards customer satisfaction, and quality service.
Client Testimonials

"The quality of research they have done for us has been excellent..."
ISO Certified


